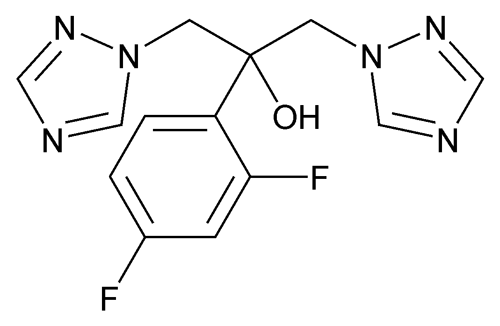
Fluconazole
C13H12F2N6O 306.27
1H-1,2,4-Triazole-1-ethanol, 1-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-ylmethyl)-;
2,4-Difluoro-1¢,1¢-bis(1H-1,2,4-triazol-1-ylmethyl)benzyl alcohol

 [86386-73-4].
[86386-73-4].
(floo kon' a zole).
C13H12F2N6O 306.27
1H-1,2,4-Triazole-1-ethanol, 1-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-ylmethyl)-;
2,4-Difluoro-1¢,1¢-bis(1H-1,2,4-triazol-1-ylmethyl)benzyl alcohol
DEFINITION
Fluconazole contains NLT 98.0% and NMT 102.0% of C13H12F2N6O, calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Sample solution:
Dissolve 200 mg of Fluconazole in 100 mL of glacial acetic acid.
Analysis:
Titrate with 0.1 N perchloric acid VS, using a suitable anhydrous electrode system. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 15.31 mg of fluconazole (C13H12F2N6O).
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
[Note—On the basis of information regarding the manufacturing process, perform either: (a) Organic Impurities, Procedure 1 or (b) Organic Impurities, Procedure 2 and Organic Impurities, Procedure 3. ]
• Organic Impurities, Procedure 1
Mobile phase:
Acetonitrile and water (20:80)
Standard solution:
10 µg/mL each of USP Fluconazole RS, USP Fluconazole Related Compound A RS, USP Fluconazole Related Compound B RS, and USP Fluconazole Related Compound C RS, dissolved in acetonitrile, and then diluted in Mobile phase
Sample solution:
3 mg/mL of Fluconazole in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 260 nm
Column:
4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature:
40
Flow rate:
0.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The retention times for fluconazole related compound A, fluconazole related compound B, fluconazole related compound C, and fluconazole are about 4.9, 8.0, 8.5, and 9.9 min, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between fluconazole related compound B and fluconazole related compound C
Relative standard deviation:
NMT 5.0% for each peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of fluconazole related compound A, fluconazole related compound B, and fluconazole related compound C in the portion of Fluconazole taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of fluconazole related compound A, fluconazole related compound B, or fluconazole related compound C from the Sample solution |
| rS | = | = average peak response of fluconazole related compound A, fluconazole related compound B, and fluconazole related compound C for replicate injections of the Standard solution |
| CS | = | = concentration of USP Fluconazole Related Compound A RS, USP Fluconazole Related Compound B RS, and USP Fluconazole Related Compound C RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluconazole in the Sample solution (mg/mL) |
Calculate the percentage of any other impurities in the portion of Fluconazole taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of any other impurity from the Sample solution |
| rS | = | = average peak response of fluconazole for replicate injections of the Standard solution |
| CS | = | = concentration of USP Fluconazole RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluconazole in the Sample solution (mg/mL) |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Fluconazole related compound A | 0.5 | 0.2 |
| Fluconazole related compound B | 0.81 | 0.1 |
| Fluconazole related compound C | 0.86 | 0.2 |
| Fluconazole | 1.0 | — |
| Specified impurity | 0.6 | 1.0 |
| Any other individual impurity | — | 0.1 |
| Total unknown impurities | — | 0.3 |
| Total impurities | — | 1.5 |
• Organic Impurities, Procedure 2
Solution A:
0.01 M anhydrous sodium acetate solution. Adjust with 1 N acetic acid to a pH of 5.0, filter, and degas.
Solution B:
Acetonitrile
Solution C:
Methanol
Mobile phase:
See Table 2.
Table 2
| Time (min) |
Solution A (%) |
Solution B (%) |
Solution C (%) |
|---|---|---|---|
| 0 | 80 | 5 | 15 |
| 10 | 80 | 5 | 15 |
| 20 | 30 | 55 | 15 |
| 23 | 30 | 55 | 15 |
| 25 | 80 | 5 | 15 |
| 30 | 80 | 5 | 15 |
Diluent:
Methanol and Solution A (16:84)
Standard solution:
0.01 mg/mL of USP Fluconazole RS in Diluent
System suitability solution:
0.02 mg/mL of USP Fluconazole RS and 6 µg/mL of USP Desacetyl Diltiazem Hydrochloride RS in Diluent
Sample solution:
2 mg/mL of Fluconazole in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 261 nm
Column:
4.0-mm × 10-cm; packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Samples:
Standard solution and System suitability solution
[Note—The relative retention times for fluconazole and desacetyl diltiazem are 1.0 and 1.2, respectively, System suitability solution. ]
Suitability requirements
Resolution:
NLT 10.0 between fluconazole and desacetyl diltiazem hydrochloride, System suitability solution
Column efficiency:
NLT 30,000 theoretical plates for the fluconazole peak, System suitability solution
Tailing factor:
NMT 1.4 for the fluconazole peak, System suitability solution
Relative standard deviation:
NMT 5.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Fluconazole taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of fluconazole from the Standard solution |
| CS | = | = concentration of USP Fluconazole RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Fluconazole in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 3) |
Acceptance criteria:
See Table 3.
Table 3
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Specified impurity | 0.17–0.37 | 0.72 | 0.1 |
| Specified impurity | 0.48–0.60 | 0.85 | 0.1 |
| Specified impurity | 0.67–0.79 | 1.21 | 0.1 |
| Specified impurity | 1.14–1.18 | 0.96 | 0.1 |
| Specified impurity | 1.20–1.32 | 0.97 | 0.1 |
| Any unspecified impurity |
— | 1.0 | 0.1 |
| Total impurities | — | — | 0.5 |
• Organic Impurities, Procedure 3
Standard solution A:
1 mg/mL of USP Fluconazole RS in methanol (2.0%)
Standard solution B:
0.1 mg/mL of USP Fluconazole RS from Standard solution A in methanol (0.2%)
Standard solution C:
0.05 mg/mL of USP Fluconazole RS from Standard solution A in methanol (0.1%)
Sample solution:
50 mg/mL of Fluconazole in methanol
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
10 µL
Developing solvent system:
Chloroform, methanol, and ammonium hydroxide (80:20:1)
Spray reagent A:
1.7 mg/mL of silver nitrate in water
Spray reagent B (potassium iodoplatinate solution):
375 mg of chloroplatinic acid in 5 mL of 1 N hydrochloric acid. Dissolve 5 g of potassium iodide in 50 mL of water, and store in a light-resistant container. Prepare a mixture of water, potassium iodide solution, and chloroplatinic acid solution (20:9:1).
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
Spray the dry plate with Spray reagent A, and expose the plate to 365-nm UV light for 10–20 min. Dry the plate for 20 min between 80 and 90
and 90 , then spray the plate with Spray reagent B. Allow the plate to dry. Examine the plate and compare the intensities of any secondary spots observed in the Sample solution with those of the principal spots in the Standard solutions.
, then spray the plate with Spray reagent B. Allow the plate to dry. Examine the plate and compare the intensities of any secondary spots observed in the Sample solution with those of the principal spots in the Standard solutions.
Acceptance criteria:
No spot from the Sample solution with an RF value between 0.10–0.25 and 0.27–0.41 is larger or more intense than that from Standard solution B (0.2%).
• Iron  241
241
Sample solution:
Transfer 0.5 g of the sample into a test tube. Dissolve in 5 mL of alcohol, and add 5 mL of distilled water.
Acceptance criteria:
NMT 20 ppm
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 0.5% of its weight.
for 3 h: it loses NMT 0.5% of its weight.
• Clarity and Color of Solution
Sample solution:
Dissolve a sample in methanol to obtain a 5-in-100 solution (w/v).
Acceptance criteria:
The solution is clear and colorless.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store below 30 .
.
• Labeling:
If a procedure for Organic Impurities other than Procedure 1 is used, then the labeling states with which Organic Impurities procedure(s) the article complies.
• USP Reference Standards  11
11
USP Desacetyl Diltiazem Hydrochloride RS
C20H24N2O3S·HCl 408.95
C20H24N2O3S·HCl 408.95
USP Fluconazole Related Compound A RS 
2-[2-Fluoro-4-(1H-1,2,4-triazol-1-yl)phenyl]-1,3-bis(1H-1,2,4-triazol-1-yl)-propan-2-ol.

2-[2-Fluoro-4-(1H-1,2,4-triazol-1-yl)phenyl]-1,3-bis(1H-1,2,4-triazol-1-yl)-propan-2-ol.
USP Fluconazole Related Compound B RS
2-(4-Fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-propan-2-ol.
2-(4-Fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-propan-2-ol.
USP Fluconazole Related Compound C RS
1,1¢-(1,3-Phenylene)di(1H-1,2,4-triazole).
1,1¢-(1,3-Phenylene)di(1H-1,2,4-triazole).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3204
Pharmacopeial Forum: Volume No. 34(1) Page 96