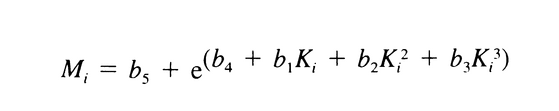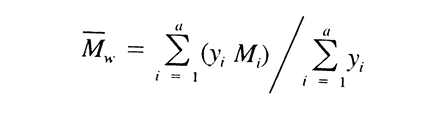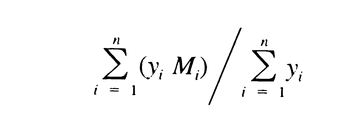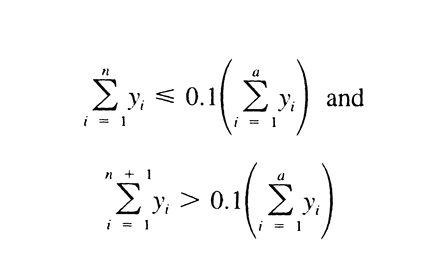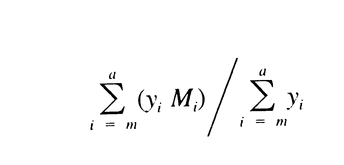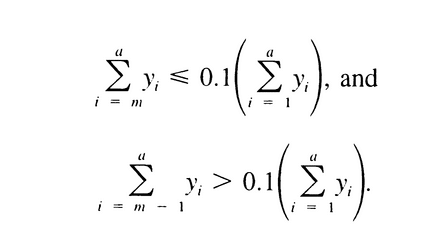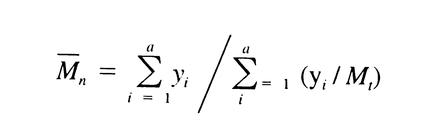Dextran 40
(dex' tran).
Dextrans.
Dextrans
» Dextran 40 is derived by controlled hydrolysis and fractionation of polysaccharides elaborated by the fermentative action of certain strains of Leuconostoc mesenteroides (NRRL, B.512 F; NCTC, 10817) on a sucrose substrate. It is a glucose polymer in which the linkages between glucose units are almost entirely of the 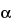 -1:6 type. Its weight average molecular weight is in the 35,000 to 45,000 range.
-1:6 type. Its weight average molecular weight is in the 35,000 to 45,000 range.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards  11
11 —
—
USP Dextran 40 RS
USP Dextran 4 Calibration RS
USP Dextran 10 Calibration RS
USP Dextran 40 Calibration RS
USP Dextran 70 Calibration RS
USP Dextran 250 Calibration RS
USP Dextran Vo Marker RS
USP Dextran 40 System Suitability RS
USP Endotoxin RS
Color of solution—
The absorbance of a solution in water (1 in 10), measured in a 4-cm cell determined at 375 nm against a water blank, is not greater than 0.20.
Identification—
B:
Prepare four Test solutions of Dextran 40 in water, in such a manner that the concentrations are accurately known and approximately evenly distributed in the range of 2% to 0.5%. Using a capillary tube viscosimeter having dimensions such that the flow time of water is not less than 100 seconds, measure the flow times of water and of the Test solution at 20 . Calculate the viscosity numbers of each of the Test solutions by the formula:
. Calculate the viscosity numbers of each of the Test solutions by the formula:
{ln[(RD)(t / t0)]} / C
in which RD is the ratio of the density of the individual Test solution to that of water; t and t0 are the flow times for the Test solution and water, respectively; and C is the concentration, in g per mL, of Dextran 40 in the Test solution. Plot the viscosity numbers of each of the Test solutions against their respective concentrations, and draw the straight line of best fit through the points and extrapolate to zero concentration: the value of the intercept is between 18 and 23 mL per g.
Specific rotation  781S
781S :
between +195
:
between +195 and +203
and +203 .
.
Test solution:
20 mg per mL, heated, if necessary, on a water bath to dissolve.
Bacterial endotoxins  85
85 (where it is labeled as intended for use in the preparation of injectables)—
When tested in Sodium Chloride Injection (1 in 10), it contains not more than 1.0 USP Endotoxin Unit per mL.
(where it is labeled as intended for use in the preparation of injectables)—
When tested in Sodium Chloride Injection (1 in 10), it contains not more than 1.0 USP Endotoxin Unit per mL.
Safety—
Inject intravenously 1.0 mL of a sterile 1 in 10 solution of 10% Dextran 40 in saline TS into each of five mice weighing 18 to 20 g. The injection period is not less than 10 seconds and not greater than 15 seconds. If there are no deaths within 72 hours, it meets the requirements of the test. If 1 or more animals die, continue the test using 10 mice weighing 20 ± 0.5 g. If all animals survive for 72 hours, the requirements of the test are met.
pH  791
791 :
between 4.5 and 7.0, in a solution (1 in 10).
:
between 4.5 and 7.0, in a solution (1 in 10).
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 5 hours: it loses not more than 7.0% of its weight.
for 5 hours: it loses not more than 7.0% of its weight.
Sulfate  221
221 —
A 1.5-g portion shows no more sulfate than corresponds to 0.45 mL of 0.020 N sulfuric acid (0.03%).
—
A 1.5-g portion shows no more sulfate than corresponds to 0.45 mL of 0.020 N sulfuric acid (0.03%).
Heavy metals, Method II  231
231 :
5 µg per g.
:
5 µg per g.
Limit of nitrogenous impurities (where it is labeled as intended for use in the preparation of injectables)—
Sulfate solution—
To 1000 mL of sulfuric acid add 5 g of anhydrous cupric sulfate and 500 g of potassium sulfate. Dissolve by heating, and store at 60 . [note—If storage at 60
. [note—If storage at 60 is not possible, prepare a smaller quantity of Sulfate solution on the day of use, adjusting the proportions accordingly. ]
is not possible, prepare a smaller quantity of Sulfate solution on the day of use, adjusting the proportions accordingly. ]
Indicator—
Dilute a mixture of 20 mL of a 0.1% solution of bromocresol green in alcohol and 4 mL of methyl red TS with water to 100 mL.
Procedure—
Transfer 0.2 g, accurately weighed, to a micro-Kjeldahl flask. Add 4 mL of Sulfate solution. Heat until the solution exhibits a clear green color and the sides of the flask are free from carbonaceous material. Cool, and transfer the solution to a steam distillation unit. Rinse the Kjeldahl flask three times with 5 mL of water, adding the washings to the solution. Add 15 mL of 45% sodium hydroxide solution, immediately close the distillation apparatus, and commence steam distillation without delay. Receive the distillate in 1 mL of Indicator in a 100-mL flask, keeping the end of the condensing tube below the liquid surface for 5 minutes and above the liquid surface for 1 minute. Upon completion of the distillation, remove the receiving flask, and rinse the end of the condensing tube with a small quantity of water, adding the rinse to the distillate. Titrate the distillate with 0.010 N hydrochloric acid until the color changes from blue to reddish violet. Perform a blank determination, and make any necessary correction. The corrected volume of 0.010 N hydrochloric acid titrated does not exceed 0.14 mL (0.01%, as N).
Limit of alcohol and related impurities—
Test solution—
Dissolve without heating 5.0 g in 100 mL of water, and distill the solution, collecting the first 45 mL of the distillate. Dilute the distillate with water to 50.0 mL, and mix.
Standard solution—
To 25.0 mL of the Test solution add 0.5 mL of a 2.5% (w/v) solution of n-propyl alcohol.
Chromatographic system—
The gas chromatograph is equipped with a flame-ionization detector and contains a 2-mm × 1.8-m column packed with support S3. The column temperature is maintained at about 160 , the injection port temperature is maintained at about 240
, the injection port temperature is maintained at about 240 , and the detector is maintained at about 210
, and the detector is maintained at about 210 . The carrier gas is nitrogen, flowing at a rate of about 25 mL per minute. [note—Injector seals may deteriorate after multiple injections of the Standard and Test solutions. Inspect the seals before making a series of injections. ]
. The carrier gas is nitrogen, flowing at a rate of about 25 mL per minute. [note—Injector seals may deteriorate after multiple injections of the Standard and Test solutions. Inspect the seals before making a series of injections. ]
Procedure—
Separately inject equal volumes (about 1 µL) of the Test solution, the Standard solution, and a 0.05% (w/v) solution of n-propyl alcohol and water, and measure the peak responses. After corrections for any impurities in the n-propyl alcohol solution and water, the total area of peaks from impurities in the Test solution does not exceed the area of the n-propyl alcohol solution peak.
Antigenic impurities (where it is labeled as intended for use in the preparation of injectables)—
Prepare a sterile solution containing 100 mg per mL in Sodium Chloride Injection. At intervals of about 48 hours, inject three 0.5-mL doses into the peritoneal cavities of each of 6 guinea pigs. At 14 days after the first intraperitoneal injection, inject 0.20 mL intravenously into each of 3 of the guinea pigs, and at 21 days treat the other 3 guinea pigs similarly. Observe the animals for 30 minutes after each intravenous injection and again 24 hours later. The animals exhibit no evidence of anaphylactoid reactions, such as coughing, bristling of hair, or respiratory distress.
Molecular weight distribution and weight and number average molecular weights—
Mobile phase—
Prepare a suitable degassed and filtered solution containing 7.1 g of anhydrous sodium sulfate per L in water.
Calibration solutions—
Separately dissolve USP Dextran 4 Calibration RS, USP Dextran 10 Calibration RS, USP Dextran 40 Calibration RS, USP Dextran 70 Calibration RS, and USP Dextran 250 Calibration RS in Mobile phase to obtain solutions each containing 20 mg per mL.
Marker solution—
Prepare a solution in Mobile phase containing 3 mg of dextrose and 3 mg of USP Dextran Vo Marker RS per mL.
System suitability solution—
Prepare a solution of USP Dextran 40 System Suitability RS in Mobile phase containing 20 mg per mL.
Test solution—
Prepare a solution of Dextran 40 in Mobile phase containing 20 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and three 7.5-mm × 30-cm columns containing packing L38, and maintained at a constant temperature. Chromatograph the Marker solution, and record the peak responses as directed for Procedure: the elution profile shows two peaks, the first due to the Vo marker, the second due to dextrose. Determine the void volume, Vo, of the system as the inflection point of the ascending part of the first peak. Determine the total volume, VT , of the system as the maximum of the second peak; the tailing factor, t, of the dextrose peak is not more than 1.3; and the relative standard deviation of the ratio Vo / VT is not more than 1%. Chromatograph each of the Calibration solutions separately, and record the peak responses as directed for Procedure. Divide each profile into at least 60 vertical sections of equal volume increments. (The actual number of sections is represented by the variable a in the equations below.) Record yi, the height above the baseline, corresponding to each value of vi, the volume eluted at that section. For each value of vi, calculate the distribution coefficient, Ki, by the formula:
)—
The liquid chromatograph is equipped with a refractive index detector and three 7.5-mm × 30-cm columns containing packing L38, and maintained at a constant temperature. Chromatograph the Marker solution, and record the peak responses as directed for Procedure: the elution profile shows two peaks, the first due to the Vo marker, the second due to dextrose. Determine the void volume, Vo, of the system as the inflection point of the ascending part of the first peak. Determine the total volume, VT , of the system as the maximum of the second peak; the tailing factor, t, of the dextrose peak is not more than 1.3; and the relative standard deviation of the ratio Vo / VT is not more than 1%. Chromatograph each of the Calibration solutions separately, and record the peak responses as directed for Procedure. Divide each profile into at least 60 vertical sections of equal volume increments. (The actual number of sections is represented by the variable a in the equations below.) Record yi, the height above the baseline, corresponding to each value of vi, the volume eluted at that section. For each value of vi, calculate the distribution coefficient, Ki, by the formula:
(vi 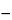 Vo) / (VT
Vo) / (VT 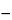 Vo).
Vo).
Find appropriate values of b1, b2, b3, b4, and b5, using a suitable method,* that, when substituted in the equation:
and the resulting values of Mi substituted, along with their corresponding values of yi, in the equation:
give values of weight average molecular weight, Mw , within 5% of the labeled values for each of the Calibration solutions and 180 ± 2 for dextrose. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure. Calculate Mw of the total molecular weight distribution using the same method as directed for the Calibration solutions, but inserting the now known values of b1, b2, b3, b4, and b5. It is between 39,000 and 46,000.
Similarly, calculate Mw of the high-fraction dextran eluted through section n by the formula:
in which n is defined by the relations:
It is between 111,000 and 135,000.
Similarly, calculate Mw of the low-fraction dextran eluted in and after section m by the formula:
in which m is defined by:
It is between 6000 and 9000.
Procedure—
Chromatograph a 50-µL volume of the Test solution, and record the peak responses. Calculate values of the weight average molecular weight, Mw , of the total molecular weight distribution of the high-fraction dextran, and of the low-fraction dextran as directed for System Suitability under Chromatography  621
621 the values are between 35,000 and 45,000, not more than 120,000, and not less than 5,000, respectively. With the values of b1, b2, b3, b4, and b5, obtained with the Calibration solutions under Chromatographic system, calculate the number average molecular weight, Mn, of the total molecular weight distribution of the Test solution by substituting the corresponding values of Mi, along with their corresponding values of yi, in the equation:
The number average molecular weight, Mn, is between 16,000 and 30,000. Where Dextran 40 is labeled as intended for use in the preparation of injectables, the ratio Mw / Mn is in the 1.4 to 1.9 range.
the values are between 35,000 and 45,000, not more than 120,000, and not less than 5,000, respectively. With the values of b1, b2, b3, b4, and b5, obtained with the Calibration solutions under Chromatographic system, calculate the number average molecular weight, Mn, of the total molecular weight distribution of the Test solution by substituting the corresponding values of Mi, along with their corresponding values of yi, in the equation:
The number average molecular weight, Mn, is between 16,000 and 30,000. Where Dextran 40 is labeled as intended for use in the preparation of injectables, the ratio Mw / Mn is in the 1.4 to 1.9 range.
*
The Gauss-Newton method, modified by Hartley [see D. Hartley Technometrics, 3 (1961)], and the G. Nilsson and K. Nilsson method [see G. Nilsson and K. Nilsson J. Chromat., 101, 137 (1974)] are suitable methods. A curve-fitting program capable of nonlinear regression may be used.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ritu Agarwal, Ph.D.
Scientific Liaison 1-301-998-6786 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 2853
Pharmacopeial Forum: Volume No. 29(6) Page 1866