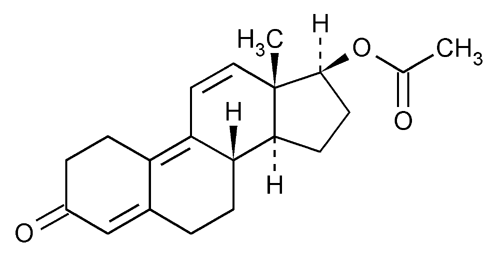
Trenbolone Acetate
Estra-4,9,11-trien-3-one, 17-(acetyloxy)-, (17
17
» Trenbolone Acetate contains not less than 97.0 percent and not more than 101.0 percent of C20H24O3.
Packaging and storage—
Preserve in tight containers, and store in a refrigerator.
Labeling—
Label it to indicate that it is for veterinary use only.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
16 µg per mL.
Medium:
Alcohol.
Absorption maxima at about 237 nm and 340 nm. Absorptivity at 340 nm is between 92.0 and 97.6.
C:
The chromatogram of the Assay preparation obtained as directed in the Assay exhibits a peak for trenbolone acetate, the retention time of which corresponds to that exhibited by the Standard preparation.
Absorbance—
The absorbance of a 1 in 10 solution of it in dehydrated alcohol, determined in a 2-cm cell at 440 nm, is not more than 0.3, dehydrated alcohol being used as the blank.
Loss on drying  731
731 —
Dry it in vacuum at 60
—
Dry it in vacuum at 60 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Chromatographic purity—
Diluent—
Prepare a mixture of chloroform and methanol (9:1).
Standard solutions—
Prepare four solutions in Diluent containing USP Trenbolone RS and USP Trenbolone Acetate RS containing in each mL 0.1 mg of each, 0.05 mg of each, 0.02 mg of each, and 0.01 mg of each, corresponding to 1.0%, 0.5%, 0.2%, and 0.1% of impurities, respectively.
Test solution—
Prepare a solution of Trenbolone Acetate in Diluent containing 10 mg per mL.
Procedure—
Separately apply 10 µL of each of the Standard solutions and the Test solution to a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture as follows. Develop the chromatograms in a solvent system consisting of a mixture of chloroform and acetone (98:2) in an unsaturated chromatographic chamber protected from light until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, dry it for about 15 seconds in a stream of dry nitrogen, and immediately develop the chromatograms a second time until the solvent front has moved about three-fourths of the length of the plate. Examine the plate under short-wavelength UV light. Spray the plate with phosphomolybdic acid TS, and heat the plate at 100
) coated with a 0.25-mm layer of chromatographic silica gel mixture as follows. Develop the chromatograms in a solvent system consisting of a mixture of chloroform and acetone (98:2) in an unsaturated chromatographic chamber protected from light until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, dry it for about 15 seconds in a stream of dry nitrogen, and immediately develop the chromatograms a second time until the solvent front has moved about three-fourths of the length of the plate. Examine the plate under short-wavelength UV light. Spray the plate with phosphomolybdic acid TS, and heat the plate at 100 for about 10 minutes. Examine the plate under visible light, and compare the intensities of any secondary spots in the chromatogram of the Test solution with those of the principal spots in the chromatograms of the Standard solutions. No trenbolone spot from the chromatogram of the Test solution is larger or more intense than the trenbolone spots from the Standard solution containing 0.1 mg of USP Trenbolone RS per mL (1%). Estimate the percentage of each other impurity observed in the chromatogram of the Test solution by comparison with the trenbolone acetate spots in the chromatograms of the Standard solutions: No other impurity spot is greater than 0.5%, and the total of all other impurities, including that of the 17
for about 10 minutes. Examine the plate under visible light, and compare the intensities of any secondary spots in the chromatogram of the Test solution with those of the principal spots in the chromatograms of the Standard solutions. No trenbolone spot from the chromatogram of the Test solution is larger or more intense than the trenbolone spots from the Standard solution containing 0.1 mg of USP Trenbolone RS per mL (1%). Estimate the percentage of each other impurity observed in the chromatogram of the Test solution by comparison with the trenbolone acetate spots in the chromatograms of the Standard solutions: No other impurity spot is greater than 0.5%, and the total of all other impurities, including that of the 17 -isomer obtained in the test for Limit of trenbolone acetate 17
-isomer obtained in the test for Limit of trenbolone acetate 17 -isomer, is not more than 1%.
-isomer, is not more than 1%.
Limit of trenbolone acetate 17 -isomer—
-isomer—
Mobile phase
, Resolution solution, and Chromatographic system—Proceed as directed in the Assay.
Standard solution—
Prepare a solution of USP Trenbolone Acetate RS in Mobile phase having a known concentration of 4 µg per mL.
Test solution—
Transfer about 20 mg of Trenbolone Acetate, accurately weighed, to a 20-mL volumetric flask, add about 10 mL of Mobile phase, swirl to dissolve, dilute with Mobile phase to volume, and mix.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Trenbolone acetate 17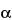 -isomer, if present, has a retention time of about 0.8 relative to that of the trenbolone acetate peak. Calculate the percentage of 17
-isomer, if present, has a retention time of about 0.8 relative to that of the trenbolone acetate peak. Calculate the percentage of 17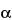 -isomer found in the Trenbolone Acetate taken by the formula:
-isomer found in the Trenbolone Acetate taken by the formula:
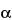 -isomer is found.
-isomer is found.
2(C / W)(ri / rS)
in which C is the concentration, in µg per mL, of USP Trenbolone Acetate RS in the Standard solution, W is the weight, in mg, of Trenbolone Acetate taken to prepare the Test solution, ri is the response of any peak at a retention time of about 0.8 in relation to that of the main trenbolone acetate peak in the chromatogram obtained from the Test solution, and rS is the peak area response of the trenbolone acetate peak in the chromatogram obtained from the Standard solution. Not more than 0.5% of the 17
Assay—
Mobile phase—
Prepare a mixture of acetonitrile and 1% ammonium acetate solution (55:45). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Resolution solution—
Prepare a solution in Mobile phase containing about 0.2 mg each of USP Trenbolone RS and USP Trenbolone Acetate RS per mL.
Standard preparation—
Prepare a solution of USP Trenbolone Acetate RS in Mobile phase having a known concentration of about 0.2 mg per mL.
Assay preparation—
Transfer about 20 mg of Trenbolone Acetate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 344-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.4 for trenbolone and 1.0 for trenbolone acetate, and the resolution, R, between the trenbolone peak and the trenbolone acetate peak is not less than 25. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 14,000 theoretical plates when calculated by the formula:
)—The liquid chromatograph is equipped with a 344-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.4 for trenbolone and 1.0 for trenbolone acetate, and the resolution, R, between the trenbolone peak and the trenbolone acetate peak is not less than 25. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 14,000 theoretical plates when calculated by the formula:
5.545(tr / Wh / 2)2
the tailing factor is not more than 1.2 when calculated by the formula:
W0.1 / 2f
in which W0.1 is the width of the peak at 10% height, and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of trenbolone acetate (C20H24O3) in the portion of Trenbolone Acetate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Trenbolone Acetate RS in the Standard preparation, and rU and rS are the trenbolone acetate peak area responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(VET05) Veterinary Drugs 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3778
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.