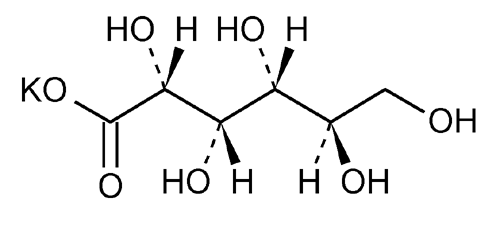
Potassium Gluconate
d-Gluconic acid, monopotassium salt.
Monopotassium d-gluconate
Monohydrate 252.26
» Potassium Gluconate is anhydrous or contains one molecule of water of hydration. It contains not less than 97.0 percent and not more than 103.0 percent of C6H11KO7, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate whether it is anhydrous or the monohydrate.
Identification—
B:
It responds to the flame test for Potassium  191
191 .
.
Loss on drying  731
731 —
Dry it in vacuum at 105
—
Dry it in vacuum at 105 for 4 hours: the anhydrous form loses not more than 3.0% of its weight, and the monohydrate loses between 6.0% and 7.5% of its weight.
for 4 hours: the anhydrous form loses not more than 3.0% of its weight, and the monohydrate loses between 6.0% and 7.5% of its weight.
Heavy metals, Method I  231
231 —
Dissolve 1 g in 10 mL of water, add 6 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 0.002%.
—
Dissolve 1 g in 10 mL of water, add 6 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 0.002%.
Reducing substances—
Transfer 1.0 g to a 250-mL conical flask, dissolve in 10 mL of water, and add 25 mL of alkaline cupric citrate TS. Cover the flask, boil gently for 5 minutes, accurately timed, and cool rapidly to room temperature. Add 25 mL of 0.6 N acetic acid, 10.0 mL of 0.1 N iodine VS, and 10 mL of 3 N hydrochloric acid, and titrate with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, omitting the specimen, and note the difference in volumes required. Each mL of the difference in volume of 0.1 N sodium thiosulfate consumed is equivalent to 2.7 mg of reducing substances (as dextrose): the limit is 1.0%.
Assay—
Potassium stock solution—
Dissolve 190.7 mg of potassium chloride, previously dried at 105 for 2 hours, in water. Transfer to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 100.0 mL of this solution to a 1000-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10 µg of potassium (equivalent to 19.07 µg of potassium chloride) per mL.
for 2 hours, in water. Transfer to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 100.0 mL of this solution to a 1000-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10 µg of potassium (equivalent to 19.07 µg of potassium chloride) per mL.
Standard preparations—
To separate 100-mL volumetric flasks transfer 10.0, 15.0, and 20.0 mL, respectively, of Potassium stock solution. To each flask add 2.0 mL of sodium chloride solution (1 in 5) and 1.0 mL of hydrochloric acid, dilute with water to volume, and mix. The Standard preparations contain, respectively, 1.0, 1.5, and 2.0 µg of potassium per mL.
Assay preparation—
Transfer about 180 mg of Potassium Gluconate, accurately weighed, to a 1000-mL volumetric flask, add water to volume, and mix. Filter a portion of the solution. Transfer 5.0 mL of the filtrate to a 100-mL volumetric flask, add 2.0 mL of sodium chloride solution (1 in 5) and 1.0 mL of hydrochloric acid, dilute with water to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the Standard preparations and the Assay preparation at the potassium emission line of 766.5 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-scattering  851
851 ) equipped with a potassium hollow-cathode lamp and an air–acetylene flame, using water as the blank. Plot the absorbance of the Standard preparation versus concentration, in µg per mL, of potassium, and draw the straight line best fitting the three plotted points. From the graph so obtained, determine the concentration, in µg per mL, of potassium in the Assay preparation. Calculate the weight, in mg, of C6H11KO7 in the Potassium Gluconate taken by the formula:
) equipped with a potassium hollow-cathode lamp and an air–acetylene flame, using water as the blank. Plot the absorbance of the Standard preparation versus concentration, in µg per mL, of potassium, and draw the straight line best fitting the three plotted points. From the graph so obtained, determine the concentration, in µg per mL, of potassium in the Assay preparation. Calculate the weight, in mg, of C6H11KO7 in the Potassium Gluconate taken by the formula:
20C(234.25 / 39.10)
in which C is the concentration, in µg per mL, of potassium in the Assay preparation, 234.25 is the molecular weight of potassium gluconate, and 39.10 is the atomic weight of potassium.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Curtis Phinney
1-301-816-8540 |
(DSN05) Dietary Supplements - Non-Botanicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3345
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.