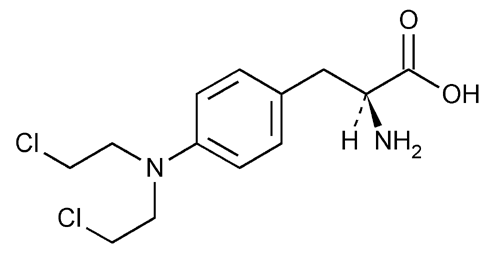
Melphalan
l-Phenylalanine, 4-bis(2-chloroethyl)amino]-.
l-3-[p-[Bis(2-chloroethyl)amino]phenyl]alanine
» Melphalan contains not less than 93.0 percent and not more than 100.5 percent of C13H18Cl2N2O2, calculated on the dried and ionizable chlorine-free basis.
Caution—Handle Melphalan with exceptional care because it is a highly potent agent.
Packaging and storage—
Preserve in tight, light-resistant, glass containers.
Identification—
B:
To 1 mL of 1 in 10,000 solution in alcohol in a glass-stoppered test tube add 1 mL of pH 4.0 acid phthalate buffer (see under Solutions in the section Reagents, Indicators, and Solutions), 1 mL of a 1 in 20 solution of 4-(p-nitrobenzyl)pyridine in acetone, and 1 mL of saline TS. Heat on a water bath at 80 for 20 minutes, and cool quickly. Add 10 mL of alcohol and 1 mL of 1 N potassium hydroxide: a violet to red-violet color is produced.
for 20 minutes, and cool quickly. Add 10 mL of alcohol and 1 mL of 1 N potassium hydroxide: a violet to red-violet color is produced.
C:
Heat 100 mg with 10 mL of 0.1 N sodium hydroxide on a water bath for 10 minutes: the resulting solution, after acidification with 2 N nitric acid, responds to the tests for Chloride  191
191 .
.
Specific rotation  781S
781S :
between
:
between  30
30 and
and  36
36 .
.
Test solution:
7 mg per mL, in methanol, prepared with the aid of gentle heating.
Loss on drying  731
731 :
Dry it in vacuum at 105
:
Dry it in vacuum at 105 to constant weight: it loses not more than 7.0% of its weight.
to constant weight: it loses not more than 7.0% of its weight.
Residue on ignition  281
281 :
not more than 0.3%.
:
not more than 0.3%.
Ionizable chlorine—
Dissolve about 500 mg of Melphalan, accurately weighed, in a mixture of 75 mL of water and 2 mL of nitric acid, allow to stand for 2 minutes, and titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically: not more than 1.0 mL of 0.1 N silver nitrate is required for each 500 mg of test specimen.
Nitrogen content  461
461 —
Determine the nitrogen content as directed under Method II, using about 325 mg of Melphalan, accurately weighed, and 0.1 N sulfuric acid VS for the titration: not less than 8.90% and not more than 9.45% of N is found, calculated on the dried basis.
—
Determine the nitrogen content as directed under Method II, using about 325 mg of Melphalan, accurately weighed, and 0.1 N sulfuric acid VS for the titration: not less than 8.90% and not more than 9.45% of N is found, calculated on the dried basis.
Assay—
Transfer to a beaker about 200 mg of Melphalan, accurately weighed, and dissolve in 20 mL of 0.5 N sodium hydroxide. Cover the beaker with a watch glass, and boil the solution for 30 minutes, adding water as necessary to maintain the volume. Cool, neutralize to phenolphthalein TS with acetic acid, and add 1 mL of acetic acid in excess. Titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically, using silver and calomel electrodes, the latter modified to contain saturated potassium sulfate solution. From the results obtained in the test for Ionizable chlorine, calculate the volume, in mL, of 0.1 N silver nitrate that is equivalent to the ionizable chlorine in the quantity of Melphalan taken for the Assay, and subtract it from the Assay titration volume. Each mL of 0.1 N silver nitrate is equivalent to 15.26 mg of C13H18Cl2N2O2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2875
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.