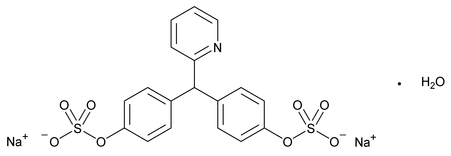
Sodium Picosulfate
Change to read:
DEFINITION
Sodium Picosulfate contains NLT 98.5% and NMT 100.5% of sodium picosulfate (C18H13NNa2O8S2), calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197
197
[Note—Methods described in Infrared Absorption  197K
197K ,
,  197M
197M , or
, or  197A
197A may be used. ]
may be used. ]
• B. Identification Tests—General, Sodium  191
191 :
Meets the requirements for the pyroantimonate precipitate test
:
Meets the requirements for the pyroantimonate precipitate test
ASSAY
• Procedure
Sample solution:
Dissolve 400 mg of Sodium Picosulfate in 80 mL of methanol.
Analysis:
Titrate with 0.1 N perchloric acid VS (see Titrimetry  541
541 ), determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 48.14 mg of sodium picosulfate (C18H13NNa2O8S2).
), determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 48.14 mg of sodium picosulfate (C18H13NNa2O8S2).
Acceptance criteria:
98.5%–100.5% on the anhydrous basis
IMPURITIES
• Chloride and Sulfate, Chloride  221
221 :
A 1.0-g portion shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid: NMT 0.02%.
:
A 1.0-g portion shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid: NMT 0.02%.
• Chloride and Sulfate, Sulfate  221
221 :
A 500-mg portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid: NMT 0.04%.
:
A 500-mg portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid: NMT 0.04%.
• Organic Impurities
Buffer:
2.3 g/L of dibasic sodium phosphate dihydrate in water. For each liter of solution, add 200 mg of cetylrimethylammonium bromide, and adjust with phosphoric acid to a pH of 7.5.
Mobile phase:
Acetonitrile and Buffer (45:55). [Note—If necessary, vary the buffer/acetonitrile proportion in 10-mL increments per L of Mobile phase in order to fulfill the resolution requirement. ]
Impurity solution:
0.025 mg/mL of USP Sodium Picosulfate Related Compound A RS in Mobile phase
System suitability solution:
0.5 mg/mL of sodium picosulfate and 0.5 µg/mL of USP Sodium Picosulfate Related Compound A RS (from Impurity solution), in Mobile phase
Sample solution:
0.5 mg/mL of Sodium Picosulfate in Mobile phase
Diluted sample solution:
0.5 µg/mL of Sodium Picosulfate in Mobile phase, from Sample solution
Sensitivity solution:
0.25 µg/mL of Sodium Picosulfate in Mobile phase, from Diluted sample solution
Chromatographic system
Mode:
LC
Detector:
UV 263 nm
Column:
4.6-mm or 4.0-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1.0 mL/min
Injection volume:
40 µL
System suitability
Samples:
System suitability solution and Sensitivity solution
Suitability requirements
Resolution:
NLT 4 between sodium picosulfate related compound A and sodium picosulfate, System suitability solution
Signal-to-noise ratio:
NLT 10, Sensitivity solution
Analysis
Samples:
Sample solution and Diluted sample solution
Calculate the percentage of each impurity in the portion of Sodium Picosulfate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response for sodium picosulfate from the Diluted sample solution |
| CS | = | = concentration of Sodium Picosulfate in the Diluted sample solution (mg/mL) |
| CU | = | = concentration of Sodium Picosulfate in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 1) |
Acceptance criteria:
See Table 1. Disregard any peak below 0.05%.
Table 1
| Name
|
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria (%) |
|---|---|---|---|
| 4,4¢-[(Pyridin-2-yl) methylene]bisphenol |
0.5 | 2.0 | 0.2 |
| 4-[(Pyridin-2-yl)(4-hydroxyphenyl)methyl]phenyl sodium sulfate (sodium picosulfate related compound A) |
0.7 | 1.4 | 0.2 |
| Sodium picosulfate | 1.0 | — | — |
| Any other individual impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 0.5 |
SPECIFIC TESTS
• Color of Solution
Standard solution:
Mix 0.75 mL of Matching Fluid O (see Color and Achromicity  631
631 ) with 99.25 mL of dilute hydrochloric acid (10 g/L).
) with 99.25 mL of dilute hydrochloric acid (10 g/L).
Sample solution:
Dissolve 2.5 g of Sodium Picosulfate in 50 mL of carbon dioxide-free water. [Note—Retain the remaining portion of the Sample solution for the test for Acidity and Alkalinity. ]
Analysis:
Proceed as directed in Color and Achromicity  631
631 .
.
Acceptance criteria:
The Sample solution is not more intensely colored than the Standard solution.
• Acidity and Alkalinity
Analysis:
To 10 mL of the portion of Sample solution retained from the test for Color of Solution add a drop of phenolphthalein TS.
Acceptance criteria:
The solution is colorless. NMT 0.25 mL of 0.01 N sodium hydroxide is required to change the color of the indicator to pink.
• Water Determination, Method Ia  921
921 :
3.0%–5.0%
:
3.0%–5.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at room temperature.
• USP Reference Standards  11
11
USP Sodium Picosulfate RS
USP Sodium Picosulfate Related Compound A RS
4-[(Pyridin-2-yl)(4-hydroxyphenyl)methyl]phenyl sodium sulfate.
C18H14NNaO5S 379.36
4-[(Pyridin-2-yl)(4-hydroxyphenyl)methyl]phenyl sodium sulfate.
C18H14NNaO5S 379.36
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Director - Chemical Medicines (301) 816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 5333
Pharmacopeial Forum: Volume No. 39(5)