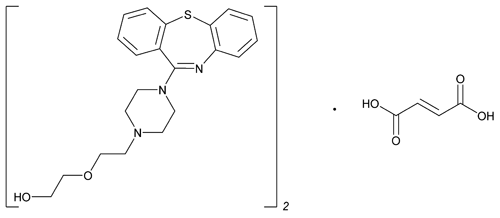
Add the following:
(kwe tye' a peen fue' ma rate).
(C21H25N3O2S)2·C4H4O4
Ethanol, 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-, (E)-2-butenedioate (2:1) (salt);
2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol fumarate (2:1) (salt)
DEFINITION
Quetiapine Fumarate contains NLT 98.0% and NMT 102.0% of quetiapine fumarate [(C21H25N3O2S)2·C4H4O4], calculated on the dried basis.
IDENTIFICATION
• B.
ASSAY
• Procedure
Buffer:
Mobile phase:
System suitability solution:
Standard stock solution:
Standard solution:
Sample stock solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Injection volume:
System suitability
Samples:
[Note—The relative retention times for quetiapine desethoxy and quetiapine are about 0.9 and 1.0, respectively.
Suitability requirements
Resolution:
Tailing factor:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of quetiapine fumarate [(C21H25N3O2S)2·C4H4O4] in the portion of Quetiapine Fumarate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Quetiapine Fumarate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Quetiapine Fumarate in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Organic Impurities
Buffer:
Solution A:
Solution B:
Diluent:
Mobile phase:
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 |
100 |
0 |
| 25 |
100 |
0 |
| 60 |
29.3 |
70.7 |
| 60.1 |
100 |
0 |
| 68 |
100 |
0 |
Peak identification solution:
System suitability solution:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Column temperature:

Flow rate:
Injection volume:
System suitability
Samples:
[Note—See Table 2 for relative retention times. Quetiapine related compound B will be the largest peak in the Peak identification solution chromatogram.
Suitability requirements
Resolution:
Tailing factor:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of any individual impurity in the portion of Quetiapine Fumarate taken:
Result = (rU/rS) × (CS/CU) × (1/F) 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of quetiapine from the Standard solution |
| CS | = | = concentration of USP Quetiapine Fumarate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Quetiapine Fumarate in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 2) |
Acceptance criteria:
Table 2
| Name |
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Fumaric acida
|
0.08 |
— |
— |
| Quetiapine quaternary saltb,c
|
0.27 |
0.62 |
0.15 |
| Quetiapine related compound G |
0.55 |
1.2 |
0.15 |
| Quetiapine related compound B |
0.67 |
1.2 |
0.15 |
| Quetiapine desethoxyd
|
0.83 |
1.0 |
0.15 |
| Quetiapine |
1.0 |
— |
— |
| Quetiapine tetraethylene glycol analogb,e |
1.2 |
1.0 |
0.10 |
| N-Ethyl quetiapineb,f
|
1.51 |
1.1 |
0.15 |
| Bis(dienzopiperazinyl) piperazineb,g
|
2.22 |
1.0 |
0.10 |
| Any other unknown individual impurity |
— |
— |
0.10 |
| Total impurities |
— |
— |
0.50 |
|
a
Peak due to counter ion, included for peak identification purposes. Not to be included in total impurities.
b
Process impurity specific to manufacturing process.
c
4-(Dibenzo[b,f][1,4]thiazepin-11-yl)-1,1-bis[2-(2-hydroxyethoxy)ethyl]piperazin-1-ium.
d
2-[4-(Dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl]ethanol.
e
2-[2-(2-{2-[4-(Dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl]ethoxy}ethoxy)ethoxy]ethanol.
f
11-(4-Ethylpiperazin-1-yl)dibenzo[b,f][1,4]thiazepine.
g
1,4-Bis(dibenzo[b,f][1,4]thiazepin-11-yl)piperazine.
|
|||
SPECIFIC TESTS
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
• USP Reference Standards  11
11
USP Quetiapine Related Compound B RS 
11-(Piperazin-1-yl)dibenzo[b,f][1,4]thiazepine.
C17H17N3S

11-(Piperazin-1-yl)dibenzo[b,f][1,4]thiazepine.
C17H17N3S
USP Quetiapine System Suitability RS 

It contains quetiapine fumarate and NLT 0.1% of each of the following impurities: Quetiapine related compound B: 11-(Piperazin-1-yl)dibenzo[b,f][1,4]thiazepine; Quetiapine related compound G: Dibenzo[b,f][1,4]thiazepin-11(10H)-one; and Quetiapine desethoxy: 2-[4-(Dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl]ethanol.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison (301) 816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 5102
Pharmacopeial Forum: Volume No. 39(6)