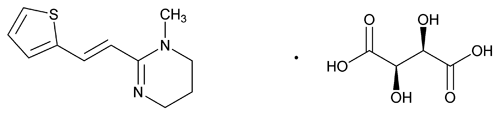Add the following:
(pi ran' tel tar' trate).
C11H14N2S·C4H6O6
Pyrimidine, 1,4,5,6-tetrahydro-1-methyl-2-[2-(2-thienyl)ethenyl]-, (E)-, (2R,3R)-2,3-dihydroxybutanedioate (1:1);
(E)-1,4,5,6-Tetrahydro-1-methyl-2-[2-(2-thienyl)vinyl]pyrimidine tartrate (1:1)
DEFINITION
Pyrantel Tartrate contains NLT 98.0% and NMT 102.0% of pyrantel tartrate (C11H14N2S·C4H6O6), calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
[Note—Concomitantly prepare potassium bromide dispersions of the Standard and sample. Differences between sets of Standard/sample spectra may occur as a result of differing specimen preparation or environmental conditions.
• C.
ASSAY
• Procedure
[Note—Protect all solutions containing Pyrantel Tartrate from light.
Mobile phase:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Injection volume:
System suitability
Sample:
Suitability requirements
Tailing factor:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of pyrantel tartrate (C11H14N2S·C4H6O6) in the portion of Pyrantel Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Pyrantel Tartrate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Pyrantel Tartrate in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Organic Impurities
[Note—Protect all solutions containing Pyrantel Tartrate from light.
Mobile phase:
Standard solution:
Sample solution:
System suitability solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Run time:
Injection volume:
System suitability
Sample:
[Note—The relative retention times for pyrantel and cis-pyrantel are 1.0 and 1.2, respectively.
Suitability requirements
System suitability requirements must be met at both 236 and 316 nm.
Resolution:
Analysis
Samples:
Calculate the percentage of each impurity detected at 236 nm in the portion of Pyrantel Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual impurity detected at 236 nm from the Sample solution |
| rS | = | = peak response detected at 236 nm from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Calculate the percentage of each impurity detected at 316 nm in the portion of Pyrantel Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual impurity detected at 316 nm from the Sample solution |
| rS | = | = peak response detected at 316 nm from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria
Any individual impurity:
Total impurities:
SPECIFIC TESTS
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
• Labeling:
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh
Scientific Liaison (301) 998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 5087
Pharmacopeial Forum: Volume No. 40(1)