Paroxetine Extended-Release Tablets
DEFINITION
Paroxetine Extended-Release Tablets contain paroxetine hydrochloride equivalent to NLT 90.0% and NMT 110.0% of the labeled amount of paroxetine (C19H20FNO3).
IDENTIFICATION
• A.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer:
3.9 g/L of ammonium acetate in water. Adjust with glacial acetic acid to a pH of 4.5.
Mobile phase:
Acetonitrile, Buffer, and triethylamine (40:60:1). Adjust with glacial acetic acid to a pH of 5.5.
Standard solution:
0.5 mg/mL of USP Paroxetine Hydrochloride RS in methanol
System suitability solution:
0.5 mg/mL of USP Paroxetine Related Compound B RS in Standard solution
Sample solution:
Nominally 0.5 mg/mL of paroxetine from NLT 10 Tablets prepared as follows. Transfer the required number of Tablets to a suitable volumetric flask. Add 80% of the flask volume of methanol. Sonicate for 30 min followed by stirring for 30 min. Dilute with methanol to volume.
Chromatographic system
Mode:
LC
Detector:
UV 295 nm
Column:
4.6-mm × 25-cm; 5-µm packing L13
Flow rate:
1 mL/min
Injection volume:
10 µL
System suitability
Samples:
Standard solution and System suitability solution
[Note—The relative retention times for paroxetine related compound B and paroxetine are 0.9 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between paroxetine related compound B and paroxetine, System suitability solution
Tailing factor:
NMT 2.0 for paroxetine, System suitability solution
Relative standard deviation:
NMT 2.0% for paroxetine, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of paroxetine (C19H20FNO3) in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Paroxetine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of paroxetine in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of paroxetine free base, 329.4 |
| Mr2 | = | = molecular weight of paroxetine hydrochloride, 365.8 |
Acceptance criteria:
90.0%–110.0%
PERFORMANCE TESTS
• Dissolution  711
711
Acid stage medium:
0.1 N hydrochloric acid; 750 mL
Buffer stage medium:
0.05 M tris buffer prepared as follows. Dissolve 6.06 g of tris(hydroxymethyl)aminomethane in 1 L of water. Add 1.8 mL of hydrochloric acid to the resulting solution. Adjust with 1000 mL of deaerated hydrochloric acid to a pH of 7.5.
Apparatus 1:
100 rpm
Times:
2 h in Acid stage; 2, 4, and 12 h in Buffer stage
Buffer and Mobile phase:
Proceed as directed in the Assay.
Acid stage standard stock solution:
0.33 mg/mL of paroxetine prepared as follows. Transfer a suitable amount of USP Paroxetine Hydrochloride RS to a suitable volumetric flask. Dissolve in 5% of the flask volume of methanol. Dilute with Acid stage medium to volume.
Acid stage standard solution:
Dilute the Acid stage standard stock solution with Acid stage medium to obtain a final concentration of (L/7500) mg/mL, where L is the label claim in mg.
Buffer stage standard stock solution:
0.25 mg/mL of paroxetine prepared as follows. Transfer a suitable amount of USP Paroxetine Hydrochloride RS to a suitable volumetric flask. Dissolve in 5% of the flask volume of methanol. Dilute with Buffer stage medium to volume.
Buffer stage standard solution:
Dilute the Buffer stage standard stock solution with Buffer stage medium to obtain a final concentration of (L/1000) mg/mL, where L is the label claim in mg.
Acid stage sample solution:
Run the Acid stage for 2 h. Withdraw 10 mL of the solution under test, and centrifuge. Use the centrifugate for analysis.
Buffer stage sample solution:
Remove the Acid stage medium from the vessel, and replace it with the Buffer stage medium. At the times specified, remove 10 mL of the solution under test, and centrifuge. Use the centrifugate for analysis.
Chromatographic system:
Proceed as directed in the Assay. For Injection volume, use 100 µL for the Acid stage analysis and 10 µL for the Buffer stage analysis.
System suitability
Samples:
Acid stage standard solution and Buffer stage standard solution
Suitability requirements
Tailing factor:
NMT 2.0, Acid stage standard solution and Buffer stage standard solution
Relative standard deviation:
NMT 3.0%, Acid stage standard solution and Buffer stage standard solution
Analysis
Samples:
Acid stage standard solution, Buffer stage standard solution, Acid stage sample solution, and Buffer stage sample solution
Calculate the percentage of the labeled amount of paroxetine (C19H20FNO3) dissolved in the Acid stage (QA):
Result = (rU/rS) × CS × (Mr1/Mr2) × V × (1/L) × 100
| rU | = | = peak response from the Acid stage sample solution |
| rS | = | = peak response from the Acid stage standard solution |
| CS | = | = concentration of paroxetine in the Acid stage standard solution (mg/mL) |
| Mr1 | = | = molecular weight of paroxetine free base, 329.4 |
| Mr2 | = | = molecular weight of paroxetine hydrochloride, 365.8 |
| V | = | = volume of Acid stage medium (750 mL) |
| L | = | = label claim (mg/Tablet) |
Calculate the concentration (Ci) of paroxetine (C19H20FNO3) dissolved at each time point in the Buffer stage:
Result = (ri/rS) × CS × (Mr1/Mr2)
| ri | = | = peak response from the Buffer stage sample solution at each time point i |
| rS | = | = peak response from the Buffer stage standard solution |
| CS | = | = concentration of the Buffer stage standard solution, mg/mL |
| Mr1 | = | = molecular weight of paroxetine free base, 329.4 |
| Mr2 | = | = molecular weight of paroxetine hydrochloride, 365.8 |
Calculate the percentage of the labeled amount (Qi) of paroxetine (C19H20FNO3) dissolved at each time point i in the Buffer stage medium:
Result1 = C1 × V × (1/L) × 100
Result2 = {[C2 × (V 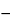 VS)] + (C1 × VS)} × (1/L) × 100
VS)] + (C1 × VS)} × (1/L) × 100
Result3 = ({C3 × [V 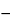 (2 × VS)]} + [(C2 + C1) × VS]) × (1/L) × 100
(2 × VS)]} + [(C2 + C1) × VS]) × (1/L) × 100
| Ci | = | = concentration of paroxetine in Buffer stage medium in the portion of sample withdrawn at time point i (mg/mL) |
| V | = | = volume of the Buffer stage medium, 1000 mL |
| L | = | = label claim (mg/Tablet) |
| VS | = | = volume of the Sample solution withdrawn from the Buffer stage medium (mL) |
Tolerances
Acid stage:
NMT 10% of the labeled amount of paroxetine (C19H20FNO3) is dissolved in 2 h.
Buffer stage:
See Table 1.
Table 1
| Time point (i) |
Time (h) |
Amount Dissolved (Tablets labeled to contain 25 mg of paroxetine) |
Amount Dissolved (Tablets labeled to contain 37.5 mg of paroxetine) |
|---|---|---|---|
| 1 | 2 | 10%–30% | 20%–45% |
| 2 | 4 | 40%–70% | 60%–85% |
| 3 | 12 | NLT 80% | NLT 80% |
The cumulative percentages of the labeled amount of paroxetine (C19H20FNO3) dissolved at the times specified conform to Acceptance Table 2 in Dissolution  711
711 .
.
• Uniformity of Dosage Units  905
905 :
Meet the requirements
:
Meet the requirements
IMPURITIES
• Organic Impurities
Solution A:
Tetrahydrofuran, water, and trifluoroacetic acid (20:180:1)
Solution B:
Acetonitrile, tetrahydrofuran, and trifluoroacetic acid (180:20:1)
Mobile phase:
See Table 2.
Table 2
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 80 | 20 |
| 30 | 80 | 20 |
| 50 | 20 | 80 |
| 60 | 20 | 80 |
| 70 | 80 | 20 |
| 80 | 80 | 20 |
System suitability solution:
1 mg/mL of USP Paroxetine Hydrochloride RS, 0.1 mg/mL of USP Paroxetine System Suitability Mixture A RS, and 1 mg/mL of USP Paroxetine Related Compound F RS in methanol. [Note—Sonication may be used to aid dissolution of the individual components. ]
Standard solution:
0.01 mg/mL of USP Paroxetine Hydrochloride RS in methanol
Sample solution:
Nominally 1 mg/mL of paroxetine from NLT 10 Tablets prepared as follows. Transfer a suitable number of Tablets to a suitable volumetric flask. Add 50% of the flask volume of methanol. Sonicate for 30 min followed by stirring for 30 min. Dilute with methanol to volume. Mix, and centrifuge. Use the clear centrifugate.
Chromatographic system
Mode:
LC
Detector:
UV 285 nm
Column:
4.6-mm × 25-cm; 5-µm packing L7
Column temperature:
40
Flow rate:
1 mL/min
Injection volume:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—See Table 3 for the relative retention times. ]
Suitability requirements
Resolution:
NLT 1.5 between paroxetine related compound A and paroxetine related compound B; NLT 1.5 between paroxetine related compound F and paroxetine, System suitability solution
Tailing factor:
NMT 2.0 for paroxetine, Standard solution
Relative standard deviation:
NMT 5.0% for paroxetine, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Paroxetine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of paroxetine in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of paroxetine free base, 329.4 |
| Mr2 | = | = molecular weight of paroxetine hydrochloride, 365.8 |
Acceptance criteria:
See Table 3.
Table 3
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Paroxetine related compound Aa | 0.67 | — |
| Paroxetine related compound Ba | 0.75 | — |
| Paroxetine related compound Fa | 0.90 | — |
| Paroxetine | 1.0 | — |
| Ethoxyparoxetineb | 1.2 | 0.2 |
| Any unspecified degradation product |
— | 0.2 |
| Total impurities | — | 0.5 |
|
a
Process impurities, included for identification only.
b
(3SR,4RS)-3-(1,3-Benzodioxol-5-yloxy)methyl)-4-(4-ethoxyphenyl)piperidine.
|
||
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers at controlled room temperature.
• USP Reference Standards  11
11
USP Paroxetine Related Compound B RS
trans-4-Phenyl-3-[(3,4-methylenedioxy)phenoxy]methylpiperidine hydrochloride; also known as [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-phenyl-, hydrochloride (3S-trans)].
trans-4-Phenyl-3-[(3,4-methylenedioxy)phenoxy]methylpiperidine hydrochloride; also known as [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-phenyl-, hydrochloride (3S-trans)].
USP Paroxetine Related Compound F RS 
trans(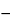 )-1-Methyl-3-[1,3-benzodioxol-5-yloxy)methyl]-4-(fluorophenyl)piperidine].
)-1-Methyl-3-[1,3-benzodioxol-5-yloxy)methyl]-4-(fluorophenyl)piperidine].
C20H22FNO3 343.39

trans(
C20H22FNO3 343.39
USP Paroxetine System Suitability Mixture A RS 
Mixture of approximately 1% paroxetine related compound A [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-methoxyphenyl)-, hydrochloride (3S-trans)] and 1% of paroxetine related compound B [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-phenyl-, hydrochloride (3S-trans)] in a matrix of paroxetine hydrochloride.

Mixture of approximately 1% paroxetine related compound A [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-methoxyphenyl)-, hydrochloride (3S-trans)] and 1% of paroxetine related compound B [piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-phenyl-, hydrochloride (3S-trans)] in a matrix of paroxetine hydrochloride.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison (301) 816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCDF2010) General Chapters - Dosage Forms | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 4769
Pharmacopeial Forum: Volume No. 39(5)



