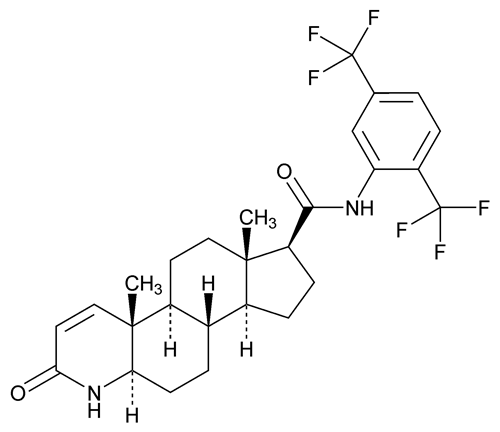
Add the following:
(doo tas' ter ide).
C27H30F6N2O2
(5
DEFINITION
Dutasteride contains NLT 97.0% and NMT 102.0% of dutasteride (C27H30F6N2O2), calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K or
or  197M
197M
• B.
ASSAY
• Procedure
Diluent:
Mobile phase:
System suitability solution:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Column temperature:

Flow rate:
Injection volume:
Run time:
System suitability
Samples:
[Note—See Table 3 for the relative retention times.
Suitability requirements
Resolution:
 -epimer and dutasteride, System Suitability solution
-epimer and dutasteride, System Suitability solution
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of dutasteride (C27H30F6N2O2) in the portion of Dutasteride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Dutasteride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Dutasteride in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Limit of Platinum
[Note—Perform this test only if platinum is a known inorganic impurity of the manufacturing process.
Diluent:
Standard stock solution:
Standard solution 1:
Standard solution 2:
Sample solution:
Instrumental conditions
(See Plasma Spectrochemistry  730
730 .)
.)
Mode:
Analytical wavelength:
Spectrophotometer system:
System suitability
Samples:
Suitability requirements
Limit of quantitation:
Result = 10 × (SD/Cs)
| SD | = | = standard deviation of platinum from the Diluent (µg/mL) |
| CS | = | = nominal concentration of platinum in the Sample solution (g/mL) |
Correlation coefficient:
Analysis
Samples:
Plot the responses of the Diluent, Standard solution 1, and Standard solution 2 versus their content (0, 0.1, and 1.0 µg/mL) of platinum. Determine the concentration, in µg/mL, of platinum in the Sample solution from the calibration curve.
Calculate the concentration, in µg/g, of platinum in the portion of Dutasteride taken:
Result = CS/CU
| CS | = | = concentration of platinum in the Sample solution (µg/mL) |
| CU | = | = concentration of Dutasteride in the Sample solution (g/mL) |
Acceptance criteria:
• Limit of Residual Solvents
Standard stock solution:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Temperatures
Column:
Table 1
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 50 |
— |
50 |
3 |
| 50 |
10 |
200 |
2 |
Injection port:

Detector:

Carrier gas:
Flow rate:
Split flow:
Septum purge:
Injector type:
Sample volume:
Sample temperature:

Equilibration time:
Thermostating time:
Needle temperature:

Transfer line temperature:

System suitability
Sample:
Suitability requirements
Resolution:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of each solvent in the portion of Dutasteride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for each solvent from the Sample solution |
| rS | = | = peak response for each solvent from the Standard solution |
| CS | = | = concentration of each solvent in the Standard solution (mg/mL) |
| CU | = | = concentration of Dutasteride in the Sample solution (mg/mL) |
Acceptance criteria:
Table 2
| Name |
Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Acetonitrile |
0.30 |
0.3 |
| Ethyl acetate |
0.60 |
0.2 |
| Dioxane |
0.83 |
0.1 |
| n-Heptane |
0.85 |
0.5 |
| Pyridine |
0.92 |
0.2 |
| Toluene |
1.0 |
0.2 |
• Organic Impurities, Procedure 1
Diluent, Mobile phase, System suitability solution, Standard solution, Sample solution, and Chromatographic system:
System suitability
Sample:
[Note—See Table 3 for the relative retention times.
Suitability requirements
Resolution:
 -epimer and dutasteride
-epimer and dutasteride
Analysis
Sample:
Calculate the percentage of each impurity in the portion of Dutasteride taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak area for each impurity from the Sample solution |
| rT | = | = total peak area from the Sample solution |
| F | = | = relative response factor (see Table 3) |
Acceptance criteria:
Table 3
| Name |
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|---|
| Dutasteride acida
|
0.10 |
1.0 |
0.2 |
|
| Dutasteride dimethylamideb |
0.11 |
1.4 |
0.2 |
|
| Dutasteride methyl esterc |
0.28 |
1.0 |
0.15 |
|
| Dutasteride ethyl esterd |
0.39 |
1.0 |
0.2 |
|
| Dutasteride 17 |
0.90 |
1.0 |
0.2 |
|
| Dutasteride 17 |
0.93 |
1.0 |
0.3 |
|
| Dutasteride |
1.00 |
— |
— |
|
| Chlorodutasterideg
|
1.15 |
0.33 |
0.4 |
|
| Dihydrodutasterideh
|
1.19 |
1.0 |
0.15 |
|
| Dutasteride 5-enei
|
1.20 |
1.0 |
0.3 |
|
| Any other individual impurity |
— |
— |
0.1 |
|
|
a
(5
b
(5
c
Methyl (5
d
Ethyl (5
e
(17
f
(5
g
(1
h
(5
i
(17
|
||||
• Organic Impurities, Procedure 2
Diluent:
Mobile phase:
System suitability solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Injection volume:
Run time:
System suitability
Sample:
Suitability requirements
Resolution:
 -dimer and dutasteride
-dimer and dutasteride  -dimer peaks
-dimer peaks
Analysis
Sample:
Integrate the dutasteride peak and all drug-related peaks eluting after the dutasteride peak.
Calculate the percentage of each impurity in the portion of Dutasteride taken:
Result = (rU/rT) × 100
| rU | = | = peak area for each impurity from the Sample solution |
| rT | = | = total peak area from the Sample solution |
Acceptance criteria:
Table 4
| Name |
Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| Dutasteride |
1.0 |
— |
|
| Dutasteride |
3.7 |
0.3 |
|
| Dutasteride |
4.3 |
0.5 |
|
| Any other individual impurity |
— |
0.1 |
|
| Total impuritiesc
|
— |
2.0 |
|
|
a
{[(5
b
{[(5
|
|||
SPECIFIC TESTS
• Water Determination, Method Ic  921
921
Sample:
Analysis:
 for 4 min.
for 4 min.
Acceptance criteria:
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
Acceptance criteria:
 to +25.0
to +25.0
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
 .
.
• USP Reference Standards  11
11
USP Dutasteride Resolution Mixture RS 
Dutasteride 17 -epimer: (5
-epimer: (5 ,17
,17 )-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide.
)-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide.
C27H30F6N2O2
Dutasteride -dimer: {[(5
-dimer: {[(5 ,17
,17 )-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide-]4-yl}{[(5
)-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide-]4-yl}{[(5 ,17
,17 )-3-oxo-4-azaandrost-1-ene]-17-yl}methanone.
)-3-oxo-4-azaandrost-1-ene]-17-yl}methanone.
C46H55F6N3O4
Dutasteride -dimer: {[(5
-dimer: {[(5 ,17
,17 )-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide-]4-yl}{[(5
)-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide-]4-yl}{[(5 ,17
,17 )-3-oxo-4-azaandrost-1-ene]-17-yl}methanone.
)-3-oxo-4-azaandrost-1-ene]-17-yl}methanone.
C46H55F6N3O4

The mixture contains the following impurities (other impurities may also be present):
Dutasteride 17
C27H30F6N2O2
Dutasteride
C46H55F6N3O4
Dutasteride
C46H55F6N3O4
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Director - Chemical Medicines (301) 998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 3247
Pharmacopeial Forum: Volume No. 39(2)