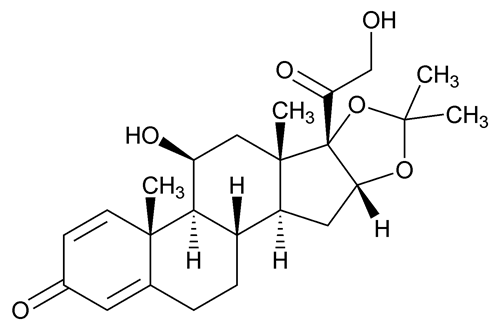
Add the following:
(des' oh nide).
C24H32O6
Pregna-1,4-diene-3,20-dione, 11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (11
11
DEFINITION
Desonide contains NLT 98.0% and NMT 102.0% of the labeled amount of desonide (C24H32O6), calculated on the dried basis.
IDENTIFICATION
• B.
ASSAY
• Procedure
Use low-actinic glassware for all solutions containing desonide.
Solution A:
Solution B:
Mobile phase:
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 |
70 |
30 |
| 4 |
68 |
32 |
| 5 |
68 |
32 |
| 9 |
65 |
35 |
| 13 |
30 |
70 |
| 16 |
30 |
70 |
Diluent:
System suitability solution:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Temperatures
Column:
 (15
(15 –22
–22 was shown to be acceptable)
was shown to be acceptable)
Autosampler:

Flow rate:
Injection volume:
System suitability
Samples:
Suitability requirements
Resolution:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of desonide (C24H32O6) in the portion of Desonide taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Desonide RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Desonide in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Organic Impurities
Solution A, Solution B, Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, and Chromatographic system:
Sensitivity solution:
System suitability
Samples:
Suitability requirements
Resolution:
Signal-to-noise ratio:
Analysis
Sample:
Calculate the percentage of each impurity in the portion of Desonide taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak area of each impurity from the Sample solution |
| rT | = | = total peak area from the Sample solution |
| F | = | = relative response factor (see Table 2) |
Acceptance criteria:
[Note—Disregard peaks that are less than 0.04% of the desonide peak from the Standard solution.
Table 2
| Name |
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|---|
| 16 |
0.31 |
1.0 |
0.15 |
|
| Prednisoloneb
|
0.48 |
1.0 |
0.15 |
|
| Desonide glyoxalc
|
0.76 |
1.0 |
0.50 |
|
| Deoxyprednisolone-16-ened
|
0.82 |
1.7 |
0.50 |
|
| Sum of desonide glyoxal and deoxyprednisolone-16-ene |
— |
— |
0.50 |
|
| Desonide |
1.00 |
— |
— |
|
| Dihydrodesonidee
|
1.07 |
0.84 |
0.50 |
|
| Epoxydesonidef
|
1.18 |
1.0 |
0.50 |
|
| Sum of dihydrodesonide and epoxydesonide |
— |
— |
0.50 |
|
| Bromodesonideg
|
1.43 |
0.68 |
0.15 |
|
| Acetyldesonideh
|
1.74 |
0.82 |
0.15 |
|
| Any other impurity |
— |
1.0 |
0.10 |
|
| Total impurities |
— |
— |
1.0 |
|
|
a
11
b
11
c
11
d
11
e
11
f
9,11
g
9
h
11
|
||||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample:
Acceptance criteria:
 to +110.0
to +110.0 , calculated on the dried basis
, calculated on the dried basis
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
• USP Reference Standards  11
11
USP Desonide RS
USP Desonide Impurities Mixture RS
This is a mixture that contains desonide and may contain about 1% each of 16 -hydroxyprednisolone, prednisolone, desonide glyoxal, deoxyprednisolone-16-ene, dihydrodesonide, epoxydesonide, bromodesonide, and acetyldesonide.
-hydroxyprednisolone, prednisolone, desonide glyoxal, deoxyprednisolone-16-ene, dihydrodesonide, epoxydesonide, bromodesonide, and acetyldesonide.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Director - Chemical Medicines (301) 998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 3024
Pharmacopeial Forum: Volume No. 39(5)