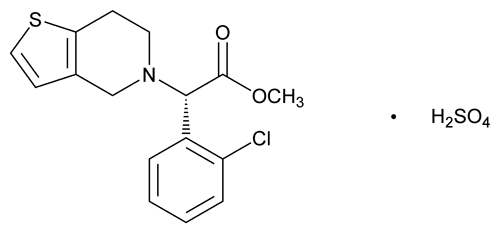
Clopidogrel Bisulfate
(kloe pid' oh grel bye sul' fate).
DEFINITION
Clopidogrel Bisulfate contains NLT 97.0% and NMT 101.5% of clopidogrel bisulfate (C16H16ClNO2S·H2SO4), calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Sulfate  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
Change to read:
• Procedure
[Note—For all clopidogrel related compounds, the concentrations are expressed as bisulfate salts. Use bisulfate salt equivalents stated on USP Reference Standards labels to calculate the concentrations as appropriate. ]
Buffer:
1.36 g/L of monobasic potassium phosphate in water
Mobile phase:
Acetonitrile and Buffer (25:75)
System suitability stock solution:
100 µg/mL of USP Clopidogrel Bisulfate RS and 200 µg/mL of USP Clopidogrel Related Compound B RS in methanol
System suitability solution:

 (ERR 1-Aug-2014)
(ERR 1-Aug-2014)

 (ERR 1-Aug-2014)
(ERR 1-Aug-2014)
Standard stock solution:
1.0 mg/mL of USP Clopidogrel Bisulfate RS in methanol
Standard solution:
0.1 mg/mL in Mobile phase from the Standard stock solution
Sample stock solution:
1 mg/mL of Clopidogrel Bisulfate in methanol
Sample solution:
0.1 mg/mL in Mobile phase, from Sample stock solution
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 15-cm; packing L57
Flow rate:
1 mL/min
Injection volume:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for the two enantiomers of clopidogrel related compound B and for clopidogrel are 0.8, 1.2, and 1.0, respectively. ]
Suitability requirements
Resolution:
Greater than 2.5 between clopidogrel and the first enantiomer of clopidogrel related compound B, System suitability solution
Relative standard deviation:
NMT 1.0% from clopidogrel bisulfate, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of clopidogrel bisulfate (C16H16ClNO2S· H2SO4) in the portion of the sample taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of Sample solution (mg/mL) |
Acceptance criteria:
97.0%–101.5% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Organic Impurities
[Note—For all clopidogrel related compounds, the concentrations are expressed as bisulfate salts. Use bisulfate salt equivalents stated on USP Reference Standards labels to calculate the concentrations as appropriate. ]
Buffer:
0.96 g/L sodium 1-pentanesulfonate. Adjust with phosphoric acid to a pH of 2.5.
Solution A:
Acetonitrile
Solution B:
Methanol
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Buffer (%) |
Solution A (%) |
Solution B (%) |
|---|---|---|---|
| 0 | 85 | 10 | 5 |
| 3 | 85 | 10 | 5 |
| 48 | 30 | 65 | 5 |
| 68 | 30 | 65 | 5 |
Diluent:
Acetonitrile and Buffer (60:40)
System suitability solution:
6.5 mg/mL of USP Clopidogrel Bisulfate RS and 0.01 mg/mL each of USP Clopidogrel Related Compound A RS and USP Clopidogrel Related Compound B RS in Diluent
Standard solution:
6.5 µg/mL of USP Clopidogrel Bisulfate RS in Diluent
Sample solution:
6.5 mg/mL of Clopidogrel Bisulfate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
3.9-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1 mL/min
Injection volume:
10 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for clopidogrel related compound A, clopidogrel, and clopidogrel related compound B are given in Table 2. ]
Suitability requirements
Peak-to-valley ratio (HP/HV):
NLT 10 where Hp is the height above the baseline of the peak due to clopidogrel related compound B and HV is the height above the baseline of the lowest point of the curve separating clopidogrel related compound B and clopidogrel, System suitability solution.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of clopidogrel related compound A, clopidogrel related compound B, and any other individual impurity in the portion of the sample taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of clopidogrel related compound A, clopidogrel related compound B, or any other impurity from the Sample solution |
| rS | = | = peak response of clopidogrel from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Clopidogrel related compound Aa | 0.4 | 0.2 |
| Clopidogrel | 1.0 | — |
| Clopidogrel related compound Bb | 1.1 | 0.3 |
| Any other impurityc | — | 0.10 |
| Total impurities | — | 0.5 |
|
a
(+)-(S)-(o-Chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid.
b
Methyl (+/
c
Disregard any peak less than 0.05%.
|
||
• Limit of Clopidogrel Related Compound C
Mobile phase:
Heptane and dehydrated alcohol (85:15)
Standard solution:
0.02 mg/mL each of USP Clopidogrel Bisulfate RS, USP Clopidogrel Related Compound B RS, and USP Clopidogrel Related Compound C RS prepared as follows. Dissolve a quantity of USP Clopidogrel Bisulfate RS, USP Clopidogrel Related Compound B RS, and USP Clopidogrel Related Compound C RS in dehydrated alcohol (about 50% of the volume of the flask), and dilute with heptane to volume.
Sample solution:
2 mg/mL of Clopidogrel Bisulfate prepared as follows. Transfer 100 mg of Clopidogrel Bisulfate to a 50-mL volumetric flask, dissolve in 25 mL of dehydrated alcohol, and dilute with heptane to volume.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; 10-µm packing L80
Flow rate:
0.8 mL/min
Injection volume:
10 µL
Run time:
1.25 times the retention time of clopidogrel
System suitability
Sample:
Standard solution
[Note—The relative retention times for clopidogrel related compound B, clopidogrel, and clopidogrel related compound C are 0.7, 1.0, and 0.6, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between clopidogrel related compound C and clopidogrel related compound B
Signal-to-noise ratio:
NLT 20 for clopidogrel related compound C peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of clopidogrel related compound C in the portion of the sample taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of clopidogrel related compound C from Sample solution |
| rS | = | = peak response of clopidogrel related compound C from Standard solution |
| CS | = | = concentration of the clopidogrel related compound C in Standard solution (mg/mL) |
| CU | = | = concentration of Clopidogrel Bisulfate in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 0.5%
SPECIFIC TESTS
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers and store at controlled room temperature.
• USP Reference Standards  11
11
USP Clopidogrel Related Compound A RS 
(+)-(S)-(o-Chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid, hydrochloride.
C15H14ClNO2S·HCl 344.26

(+)-(S)-(o-Chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid, hydrochloride.
C15H14ClNO2S·HCl 344.26
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D., MBA
Senior Scientific Liaison (301) 816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 2899
Pharmacopeial Forum: Volume No. 38(1)