Albuterol Extended-Release Tablets
DEFINITION
Albuterol Extended-Release Tablets contain albuterol sulfate equivalent to NLT 90.0% and NMT 110.0% of the labeled amount of albuterol (C13H21NO3).
IDENTIFICATION
• A.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• B. Ultraviolet Absorption  197U
197U
Standard solution:
80 µg/mL of albuterol from USP Albuterol Sulfate RS in methanol
Sample solution:
80 µg/mL of albuterol in methanol, prepared as follows. Transfer a suitable number of Tablets to a volumetric flask, and dilute with methanol to volume. Stir for 30 min, and centrifuge.
Wavelength range:
210–350 nm
Cell path:
0.2 cm
Acceptance criteria:
The Sample solution exhibits maxima and minima only at the same wavelengths as the Standard solution.
ASSAY
• Procedure
Buffer:
0.65 g/L of sodium 1-octane sulfonate and 21.7 g/L of ammonium acetate in water
Mobile phase:
Glacial acetic acid, 2-propanol, methanol, and Buffer (4:3:1:92)
Diluent:
10 mL/L of triethylamine in water
Standard stock solution:
0.2 mg/mL of USP Albuterol Sulfate RS in Diluent
Standard solution:
0.02 mg/mL of USP Albuterol Sulfate RS in Diluent, from the Standard stock solution. Transfer an aliquot of the Standard stock solution to a suitable volumetric flask, and add 4% of the flask volume of methanol. Allow to cool to room temperature, and dilute with Diluent to volume.
Sample solution:
Nominally 0.016 mg/mL of albuterol, prepared as follows. Transfer Tablets (NLT 10) to a suitable volumetric flask, add 10% of the flask volume of methanol, and sonicate for 30 min with regular swirling. Add 60% of the flask volume of Diluent, and sonicate for 30 min with swirling. Stir for 60 min, allow the solution to cool to room temperature, and dilute with Diluent to volume. Centrifuge a portion of this solution at 2500 rpm for 15 min. Transfer 10 mL of the supernatant into a 50-mL volumetric flask, add 1 mL of methanol, cool to room temperature, and dilute with Diluent to volume. Pass the solution though a 1-µm glass fiber or equivalent filter, and discard the first 3 mL of the filtrate.
Chromatographic system
Mode:
LC
Detector:
UV 276 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
1 mL/min
Injection volume:
40 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 2000 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of albuterol (C13H21NO3) in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × (M × Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Albuterol Sulfate RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
| M | = | = moles of albuterol per mole of albuterol sulfate, 2 |
| Mr1 | = | = molecular weight of albuterol, 239.31 |
| Mr2 | = | = molecular weight of albuterol sulfate, 576.70 |
Acceptance criteria:
90.0%–110.0%
PERFORMANCE TESTS
• Dissolution  711
711
Medium:
0.1 N hydrochloric acid; 900 mL
Apparatus 2:
50 rpm, with helix sinker
Time:
1, 2, 4, and 9 h
Buffer, Mobile phase, Chromatographic system, and System suitability:
Proceed as directed in the Assay, except to use an Injection volume of 50 µL.
Standard stock solution:
0.2 mg/mL of albuterol, using USP Albuterol Sulfate RS, in Medium
Standard solution:
Dilute the Standard stock solution with Medium to obtain a final concentration of (L/1000) mg/mL of albuterol, where L is the label claim in mg/Tablet of albuterol.
Sample solution:
Pass a portion of the solution under test through a suitable filter.
Analysis
Samples:
Standard solution and Sample solution
Calculate the concentration (Ci) of albuterol (C13H21NO3) in the sample withdrawn from the vessel at each time point (i):
Resulti = (rU/rS) × CS
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of albuterol in the Standard solution (mg/mL) |
Calculate the percentage of the labeled amount of albuterol (C13H21NO3) released at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = {[C2 × (V 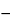 VS)] + [C1 × VS]} × (1/L) × 100
VS)] + [C1 × VS]} × (1/L) × 100
Result3 = {[C3 × (V 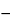 (2 × VS))] + [(C2 + C1) × VS]} × (1/L) × 100
(2 × VS))] + [(C2 + C1) × VS]} × (1/L) × 100
Result4 = {[C4 × (V 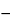 (3 × VS))] + [(C3 + C2 + C1) × VS]} × (1/L) × 100
(3 × VS))] + [(C3 + C2 + C1) × VS]} × (1/L) × 100
| Ci | = | = concentration of albuterol in the portion of sample withdrawn at time point i (mg/mL) |
| V | = | = volume of the Medium (900 mL) |
| L | = | = label claim (mg/Tablet) |
| VS | = | = volume of the Sample solution withdrawn from the Medium (mL) |
Tolerances:
See Table 1.
Table 1
| Time point (i) |
Time (h) |
Amount released (%) |
|---|---|---|
| 1 | 1 | 25–45 |
| 2 | 2 | 45–65 |
| 3 | 4 | 65–85 |
| 4 | 9 | NLT 80 |
The cumulative percentages of the labeled amount of albuterol (C13H21NO3), released at the times specified, conform to Acceptance Table 2 in Dissolution  711
711 .
.
• Uniformity of Dosage Units  905
905 :
Meet the requirements
:
Meet the requirements
IMPURITIES
• Organic Impurities, Procedure 1
Buffer:
6.8 g/L of monobasic potassium phosphate in water. Adjust with hydrochloric acid to a pH of 3.0.
Mobile phase:
Methanol and Buffer (5:95)
System suitability solution:
2 µg/mL each of USP Albuterol Sulfate RS and USP Albuterol Related Compound B RS in Mobile phase
Standard solution:
2.4 µg/mL of USP Albuterol Sulfate RS, 0.80 µg/mL of USP Levalbuterol Related Compound C RS, and 0.25 µg/mL of USP Levalbuterol Related Compound D RS (equivalent to 0.20 µg/mL free base) in Mobile phase
Sensitivity solution:
0.06 µg/mL of USP Albuterol Sulfate RS in Mobile phase
Sample solution:
Transfer an amount of powder from NLT 20 Tablets to a suitable volumetric flask to obtain a solution with a final nominal concentration of 0.2 mg/mL of albuterol. Add 70% of the flask volume of Mobile phase, and sonicate for 10 min. Stir for 30 min, and dilute with Mobile phase to volume. Pass the solution though a 1-µm glass fiber or equivalent filter, and discard the first 3 mL of the filtrate.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; 5-µm packing L11
Column temperature:
30
Flow rate:
1.5 mL/min
Injection volume:
80 µL
Run time:
5 times the retention time of albuterol
System suitability
Samples:
System suitability solution and Standard solution
[Note—Identify the impurities using the relative retention times shown in Table 2. ]
Suitability requirements:
Tailing factor:
NMT 2.0 for each compound, Standard solution
Resolution:
NLT 2 between albuterol and albuterol related compound B, System suitability solution
Relative standard deviation:
NMT 10.0% for each compound, Standard solution
Analysis
Calculate the percentage of levalbuterol related compound D in the portion of Tablets taken:
Calculate the percentage of any other impurity in the portion of Tablets taken:
Samples:
Standard solution, Sensitivity solution, and Sample solution
[Note—Identify the impurities using the relative retention times shown in Table 2. ]
Calculate the percentage of levalbuterol related compound C in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of levalbuterol related compound C from the Sample solution |
| rS | = | = peak response of levalbuterol related compound C from the Standard solution |
| CS | = | = concentration of USP Levalbuterol Related Compound C RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of levalbuterol related compound D from the Sample solution |
| rS | = | = peak response of the levalbuterol related compound D from the Standard solution |
| CS | = | = concentration of USP Levalbuterol Related Compound D RS (as the free base) in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
Result = (rU/rS) × (CS/CU) × (M × Mr1/Mr2) × 100
| rU | = | = peak response of the impurity from the Sample solution |
| rS | = | = peak response of albuterol from the Standard solution |
| CS | = | = concentration of USP Albuterol Sulfate RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
| M | = | = moles of albuterol per mole of albuterol sulfate, 2 |
| Mr1 | = | = molecular weight of albuterol, 239.31 |
| Mr2 | = | = molecular weight of albuterol sulfate, 576.70 |
Acceptance criteria:
See Table 2. Disregard peaks eluting after levalbuterol related compound C or with areas less than that of the Sensitivity solution.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Albuterol related compound Ba | 0.88 | —b |
| Albuterol | 1.0 | — |
| Chloroalbuteronec | 1.7 | —b |
| Chloroalbuterold | 2.5 | —b |
| Albuterol related compound Ae | 2.7 | —b |
| Levalbuterol related compound Df | 3.2 | 0.1 |
| Levalbuterol related compound Cg,h | 3.5 | 0.4 |
| Any other individual unspecified impurity | — | 0.2 |
|
a
2-(tert-Butylamino)-1-[4-hydroxy-3-(hydroxymethyl)phenyl]ethanone.
b
Process impurity included in the table for identification only. Process impurities are controlled in the drug substance, and are not to be reported or included in the total impurities for the drug product.
c
2-(tert-Butylamino)-1-[3-chloro-4-hydroxy-5-(hydroxymethyl)phenyl]ethanone.
d
4-[2-(tert-Butylamino)-1-hydroxyethyl]-2-chloro-6-(hydroxymethyl)phenol.
e
4-{2-[(1,1-Dimethylethyl)amino]-1-hydroxyethyl}-2-methylphenol.
f
5-[2-{(1,1-Dimethylethyl)amino}-1-hydroxyethyl]-2-hydroxy-benzaldehyde.
g
h
Disregard peaks eluting after levalbuterol related compound C.
|
||
• Organic Impurities, Procedure 2
Buffer:
6.8 g/L of monobasic potassium phosphate in water. Adjust with hydrochloric acid to a pH of 3.0.
Mobile phase:
Methanol and Buffer (30:70)
Diluent:
Methanol and Buffer (5:95)
Peak identification solution:
2.4 µg/mL of USP Albuterol Sulfate RS, 1.0 µg/mL of USP Levalbuterol Related Compound C RS, and 1.2 µg/mL of USP Levalbuterol Related Compound D RS in Diluent
Standard solution:
2.4 µg/mL of USP Albuterol Sulfate RS and 1.0 µg/mL of USP Albuterol Related Compound E RS in Diluent
Sensitivity solution:
0.06 µg/mL of USP Albuterol Sulfate RS in Diluent from Standard solution
Sample solution:
Transfer an amount of powder from NLT 20 Tablets to a suitable volumetric flask to obtain a solution with a final concentration of 0.2 mg/mL of albuterol. Add 70% of the flask volume of Diluent, and sonicate for 10 min. Stir for 30 min, and dilute with Diluent to volume. Pass the solution though a glass fiber or equivalent filter of 1-µm pore size, and discard the first 3 mL of the filtrate.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; 5-µm packing L11
Flow rate:
0.7 mL/min
Injection volume:
80 µL
Run time:
5 times the retention time of albuterol
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0 for each compound
Relative standard deviation:
NMT 10.0% for each compound
Analysis
Calculate the percentage of any other impurity in the portion of Tablets taken:
Samples:
Standard solution, Peak identification solution, Sensitivity solution, and Sample solution
Calculate the percentage of albuterol related compound E in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of albuterol related compound E from the Sample solution |
| rS | = | = peak response of albuterol related compound E from the Standard solution |
| CS | = | = concentration of USP Albuterol Related Compound E RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
Result = (rU/rS) × (CS/CU) × (M × Mr1/Mr2) × 100
| rU | = | = peak response of the impurity from the Sample solution |
| rS | = | = peak response of albuterol from the Standard solution |
| CS | = | = concentration of USP Albuterol Sulfate RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of albuterol in the Sample solution (mg/mL) |
| M | = | = moles of albuterol per mole of albuterol sulfate, 2 |
| Mr1 | = | = molecular weight of albuterol, 239.31 |
| Mr2 | = | = molecular weight of albuterol sulfate, 576.70 |
Acceptance criteria:
See Table 3. Disregard the levalbuterol related compound C peak and any peak eluting before it. Disregard peaks with areas less than that of the Sensitivity solution.
Table 3
| Compound | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Albuterol | 1.0 | — |
| Levalbuterol related compound C | 1.79 | — |
| Levalbuterol related compound D | 1.83 | — |
| Albuterol related compound Ea |
3.67 | 0.5 |
| Any other individual unspecified impurity |
— | 0.2 |
| Total impurities | — | 1.5b |
|
a
2,2'-Oxybis(methylene)bis{4-[2-(tert-butylamino)-1-hydroxyethyl]phenol}.
b
From the sum of the impurities in Procedure 1 and Procedure 2.
|
||
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at controlled room temperature.
• USP Reference Standards  11
11
USP Albuterol Related Compound B RS 
2-(tert-Butylamino)-1-[4-hydroxy-3-(hydroxymethyl)phenyl]ethanone.
C13H19NO3 237.29

2-(tert-Butylamino)-1-[4-hydroxy-3-(hydroxymethyl)phenyl]ethanone.
C13H19NO3 237.29
USP Albuterol Related Compound E RS 
2,2¢-Oxybis(methylene)bis{4-[2-(tert-butylamino)-1-hydroxyethyl]phenol}.
C26H40N2O5 460.61

2,2¢-Oxybis(methylene)bis{4-[2-(tert-butylamino)-1-hydroxyethyl]phenol}.
C26H40N2O5 460.61
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Director - Chemical Medicines (301) 998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCDF2010) General Chapters - Dosage Forms | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 2068
Pharmacopeial Forum: Volume No. 38(4)



