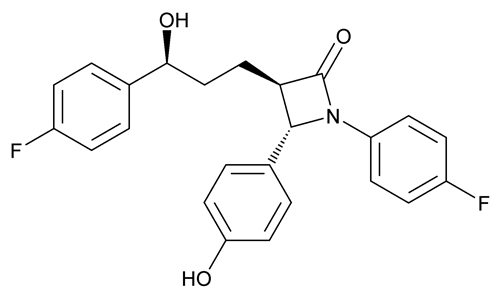
Add the following:
(e zet' i mibe).
C24H21F2NO3
2-Azetidinone, 1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-, [3R-[3
(3R,4S)-1-(p-Fluorophenyl)-3-[(3S)-3-(p-fluorophenyl)-3-hydroxypropyl]-4-(p-hydroxyphenyl)-2-azetidinone;
(3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-azetidin-2-one
DEFINITION
Ezetimibe contains NLT 98.0% and NMT 102.0% of ezetimibe (C24H21F2NO3), calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• B.
ASSAY
• Procedure
Solution A:
Solution B:
Solution C:
Mobile phase:
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
Solution C (%) |
|---|---|---|---|
| 0.0 |
63 |
27 |
10 |
| 37.0 |
63 |
27 |
10 |
| 60.0 |
40 |
50 |
10 |
| 70.0 |
40 |
50 |
10 |
| 80.0 |
10 |
80 |
10 |
| 90.0 |
10 |
80 |
10 |
| 90.1 |
63 |
27 |
10 |
| 100.0 |
63 |
27 |
10 |
Diluent:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detectors
0–5 min:
5–100 min:
Column:
Flow rate:
Injection volume:
System suitability
Sample:
Suitability requirements
Tailing factor:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of ezetimibe (C24H21F2NO3) in the portion of Ezetimibe taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of ezetimibe from the Sample solution |
| rS | = | = peak response of ezetimibe from the Standard solution |
| CS | = | = concentration of USP Ezetimibe RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Ezetimibe in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Organic Impurities, Procedure 1
Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system:
System suitability solution:
Sensitivity solution:
System suitability
Samples:
Suitability requirements
Resolution:
Tailing factor:
Relative standard deviation:
Analysis
Sample:
Calculate the percentage of each impurity in the portion of Ezetimibe taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of all peak responses from the Sample solution |
| F | = | = relative response factor from Table 2 |
Acceptance criteria:
Table 2
| Name |
Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Desfluoroaniline analoga |
0.72 |
1.0 |
0.2 |
| Ezetimibe diastereomersb |
0.77 |
1.0 |
— |
| o-Fluorobenzene isomerc
|
0.89 |
1.0 |
0.2 |
| Ezetimibe |
1.0 |
— |
— |
| m-Flouroaniline analogd |
1.13 |
1.0 |
0.2 |
| Ezetimibe ketonee
|
1.42 |
1.5 |
0.1 |
| Any unspecified impurity |
— |
1.0 |
0.10 |
| Total achiral impurities |
— |
— |
0.6 |
|
a
(3R,4S)-3-[(S)-3-(4-Fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-1-phenylazetidin-2-one.
b
The two ezetimibe diastereomers, R,R,R- and S,S,S-, elute as one peak and are quantitated using Procedure 2.
c
(3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(2-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
d
(3R,4S)-1-(3-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
e
(3R,4S)-1-(4-Fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
|
|||
• Organic Impurities, Procedure 2
Mobile phase:
Diluent:
System suitability solution:
Sensitivity solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Column temperature:

Flow rate:
Injection volume:
Run time:
System suitability
Samples:
Suitability requirements
Resolution:
Tailing factor:
Relative standard deviation:
Analysis
Sample:
Calculate the percentage of each enantiomeric or diastereomeric impurity in the portion of Ezetimibe taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of all peak responses from the Sample solution |
Acceptance criteria:
Table 3
| Name |
Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| S,S,S-Ezetimibea
|
0.76 |
0.2 |
| R,R,R-Ezetimibeb
|
0.82 |
0.1 |
| Desfluoroaniline analogc
|
0.89 |
— |
| R,R,S-Ezetimibed
|
0.93 |
0.4 |
| Ezetimibe |
1.00 |
— |
| S,S,R-Ezetimibee
|
1.12 |
0.1 |
| R,S,R-Ezetimibef
|
1.22 |
0.1 |
| Total chiral impurities |
— |
0.5 |
| Total impuritiesg
|
— |
0.9 |
|
a
(3S,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
b
(3R,4R)-1-(4-Fluorophenyl)-3-[(R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
c
Desfluoroaniline analog impurity is quantitated using Procedure 1.
d
(3R,4S)-1-(4-Fluorophenyl)-3-[(R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
e
(3S,4R)-1-(4-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
f
(3S,4R)-1-(4-Fluorophenyl)-3-[(R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
g
Include all impurities from Procedure 1 and Procedure 2.
|
||
SPECIFIC TESTS
• Water Determination, Method Ia or Method Ic  921
921 :
:
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
Temperature:

Acceptance criteria:
 25.0
25.0 to
to  30.0
30.0
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
 .
.
• USP Reference Standards  11
11
USP Ezetimibe System Suitability Mixture RS
(3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(2-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one;
C24H21F2NO3
((3R,4S)-1-(4-Fluorophenyl)-3-[(R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one).
C24H21F2NO3
It contains ezetimibe, o-fluorobenzene isomer
(3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(2-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one;
C24H21F2NO3
and R,R,S-ezetimibe
((3R,4S)-1-(4-Fluorophenyl)-3-[(R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one).
C24H21F2NO3
[Note—It may also contain desfluoroaniline analog.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D., MBA
Senior Scientific Liaison (301) 816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Supplement : No. 2 Page 8082
Pharmacopeial Forum: Volume No. 40(5)