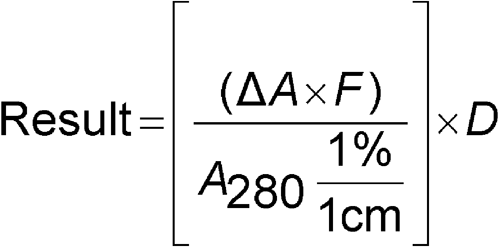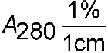Add the following:
INTRODUCTION
The purpose of this chapter is to describe the quality attributes and associated tests with acceptance criteria for enzymatic preparations used in biopharmaceutical manufacturing. The quality of ancillary materials, including enzymes, used in biopharmaceutical manufacturing can have an impact on the therapeutic products. Several enzymes are used in this type of cell processing. Examples include trypsin, collagenase, pepsin, and papain. This chapter does not discuss applications of these enzymes but rather focuses on tests to assess their quality as process materials.
DEFINITION
Recombinant trypsin, a key raw material in biopharmaceutical manufacturing, is a serine protease that cleaves peptide chains mainly at the carboxyl end of the amino acids arginine and lysine. The amino acid sequence of recombinant trypsin is identical to that of trypsin from porcine pancreas, and the recombinant trypsin is produced by methods based on recombinant DNA technology in the yeast Pichia pastoris. Therefore, the specifications described in this chapter apply only to recombinant porcine trypsin produced in yeast. Because of the recombinant production process, recombinant trypsin is free of chymotrypsin. Two active forms of trypsin are known:  -trypsin (23,463 daltons) and
-trypsin (23,463 daltons) and  -trypsin (23,481 daltons). Autolysis of
-trypsin (23,481 daltons). Autolysis of  -trypsin at the peptide bond between Arg99 and Val100, Lys125 and Ser126, or Lys139 and Ala140 results in three possible isoforms of
-trypsin at the peptide bond between Arg99 and Val100, Lys125 and Ser126, or Lys139 and Ala140 results in three possible isoforms of  -trypsin. All isoforms are held together by disulfide bridges and remain correctly folded. As a consequence of hydrolysis of a peptide bond, the molecular weight of
-trypsin. All isoforms are held together by disulfide bridges and remain correctly folded. As a consequence of hydrolysis of a peptide bond, the molecular weight of  -trypsin is more than that of
-trypsin is more than that of  -trypsin by 18 daltons. The peak area for
-trypsin by 18 daltons. The peak area for  -trypsin is NLT 70%, and the peak area for
-trypsin is NLT 70%, and the peak area for  -trypsin is NMT 20% as determined by the HPLC procedure described in the test for Purity. The specific activity is NLT 180 Units/mg of protein using carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate as the substrate described in the Assay.
-trypsin is NMT 20% as determined by the HPLC procedure described in the test for Purity. The specific activity is NLT 180 Units/mg of protein using carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate as the substrate described in the Assay.
[Note—One Unit of trypsin activity using carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate as the substrate corresponds to 21 USP Trypsin Units. One USP Trypsin Unit is the activity causing a change in absorbance of 0.003 per minute under the conditions specified in the Assay in the Crystallized Trypsin monograph using N-benzoyl-l-arginine ethyl ester hydrochloride as the substrate. Therefore, the specific activity of 180 Units/mg of protein using carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate as the substrate for recombinant trypsin corresponds to 3800 USP Trypsin Units/mg of protein.
IDENTIFICATION
• A.
• B.
 -trypsin in the Sample solution corresponds to that of the Standard solution, as obtained in the test for Purity.
-trypsin in the Sample solution corresponds to that of the Standard solution, as obtained in the test for Purity.
ASSAY
• Procedure
Buffer:
 ).
).
Substrate stock solution:
Substrate solution:
Standard solutions:
 . Start preparing Standard solutions immediately when the temperature has reached 4
. Start preparing Standard solutions immediately when the temperature has reached 4 . Prepare each Standard solution by diluting USP Trypsin Recombinant Porcine RS with water at 1:68,921 dilution. Prepare at least five Standard solutions in parallel. Assay each Standard solution in duplicate. [Note—Use an adjustable pipettor for each measurement and dilution operation. Use polystyrene test tubes for preparing Standard solutions and Sample solutions, and use polystyrene pipet tips containing polyethylene filters to transfer samples. The pipet tip should not be wet before transfer, and each pipet tip should be used only for transferring one sample.
. Prepare each Standard solution by diluting USP Trypsin Recombinant Porcine RS with water at 1:68,921 dilution. Prepare at least five Standard solutions in parallel. Assay each Standard solution in duplicate. [Note—Use an adjustable pipettor for each measurement and dilution operation. Use polystyrene test tubes for preparing Standard solutions and Sample solutions, and use polystyrene pipet tips containing polyethylene filters to transfer samples. The pipet tip should not be wet before transfer, and each pipet tip should be used only for transferring one sample.
Sample solutions:
Instrumental conditions
Mode:
Analytical wavelength:
Pathlength:
Temperature:

Analysis
Samples:
Transfer 1.10 mL of Substrate solution into a polystyrene semimicro cuvette, allow the temperature to stabilize, check the cuvette for the specified temperature, and wait for 10 min. Start the reaction by adding 0.020 mL of Standard solution or Sample solution. Record the absorbance for at least 5 min, and determine the change in absorbance (DA/min) from the linear range of the reaction.
Calculate the activity of recombinant trypsin in Units/mL:
Result = [VT/( × V × B)] × (DA/min) × D
× V × B)] × (DA/min) × D
| VT | = | = volume of the reaction mixture, 1.12 mL |
| = | = extinction coefficient for 405 nm, 10.4 (mmol |
|
| V | = | = volume of Standard solution or Sample solution, 0.020 mL |
| B | = | = absorption pathlength, 1 cm |
| D | = | = dilution factor |
[Note—One Unit will release the equivalent of 1 mmol of 4-nitril aniline from carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate per min under the conditions of the Assay.
Calculate the specific activity of recombinant trypsin in Units/mg of protein:
Result = Activity/C
| C | = | = protein concentration of recombinant trypsin (mg/mL) |
System suitability
Samples:
Suitability requirement:
Acceptance criteria
Specific activity:
Relative standard deviation:
PURITY
• Procedure
Solution A:
Solution B:
Mobile phase:
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 |
75 |
25 |
| 25 |
55 |
45 |
| 30 |
10 |
90 |
| 34 |
10 |
90 |
| 35 |
75 |
25 |
| 45 |
75 |
25 |
Standard solution:
Sample solution:
[Note—Keep Standard solution and Sample solution at 2 –8
–8 if they are not ready to be injected immediately after preparation.
if they are not ready to be injected immediately after preparation.
Chromatographic system
Mode:
Detector:
Column:
Column temperature:

Flow rate:
Injection volume:
System suitability
Sample:
[Note—The retention time for the main peak of recombinant trypsin is 12–17 min.
Suitability requirements
Resolution:
 -trypsin and
-trypsin and  -trypsin
-trypsin
Analysis
Sample:
Record the chromatograms, and measure the peak areas. Evaluate the purity of trypsin using the area-% method. Time of integration is 25 min. The blank should be considered for integration. Peaks that elute as a fronting shoulder of  -trypsin are integrated by perpendicular dropping if and only if a minimum is formed. Peaks that elute as a tailing shoulder of
-trypsin are integrated by perpendicular dropping if and only if a minimum is formed. Peaks that elute as a tailing shoulder of  -trypsin are evaluated by tangential integration if and only if a minimum is formed.
-trypsin are evaluated by tangential integration if and only if a minimum is formed.
Acceptance criteria:
 -trypsin and NMT 20% for the peak area of
-trypsin and NMT 20% for the peak area of  -trypsin
-trypsin
SPECIFIC TESTS
• Protein Content
4 N hydrochloric acid solution:
Storage buffer:
Sample solutions:
Blank solution:
Instrumental conditions
Mode:
Analytical wavelength:
Pathlength:
System suitability
Sample:
Suitability requirement:
Analysis
Samples:
Calculate the protein concentration in mg/mL:
DA = AU  AB
AB
• Microbial Enumeration Tests  61
61 :
:
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
 15
15 to
to  25
25 .
.
• Labeling:
• USP Reference Standards  11
11
USP Trypsin Recombinant Porcine RS
1
A suitable carbobenzoxy-valyl-glycyl-arginine-4-nitril-anilide acetate is Chromozym TRY from Roche Applied Science (catalog number 10378496103) or equivalent.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Edith Chang, Ph.D.
Scientific Liaison (301) 816-8392 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 164
Pharmacopeial Forum: Volume No. 39(5)