INTRODUCTION
This general test chapter provides a procedure and acceptance criterion (limit) to control the principal degradation product of acetaminophen, 4-aminophenol, an impurity that can form by hydrolysis of acetaminophen.
SOLUTION PREPARATIONS
All solution preparations that contain acetaminophen or 4-aminophenol should be protected from light and should be stored only for as long as can be supported by solution stability data acquired during verification under actual conditions of use.
Buffer:
4.0 g/L of sodium citrate dihydrate and 1.5 g/L of anhydrous citric acid, in water.
Diluent:
Buffer and acetonitrile (9:1)
Solution A:
10 mM phosphate buffer prepared by adding 0.60 g of monobasic potassium phosphate and 0.82 g of dibasic sodium phosphate to a 1-L volumetric flask, dissolving and diluting with water to volume to a pH of 7.0
Solution B:
Water
Solution C:
Acetonitrile
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
Solution C (%) |
|---|---|---|---|
| 0 | 90 | 5 | 5 |
| 5 | 90 | 5 | 5 |
| 7 | 10 | 10 | 80 |
| 7.1 | 90 | 5 | 5 |
| 10 | 90 | 5 | 5 |
Standard stock solution:
25 µg/mL of USP 4-Aminophenol RS in Diluent. Prepare fresh in conjunction with the other solution preparations described below. Discard after 4 h or as supported by solution stability data. [Note—See Chromatographic Adjustments, solution stability, below. ]
System suitability solution:
2.5 µg/mL of USP 4-Aminophenol RS from the Standard stock solution in Diluent
Sample stock solution:
Nominally 10 mg/mL of acetaminophen from a suitable quantity of drug product in Diluent. [Note—Either component of the Diluent may be introduced to the drug product first, followed by addition of the other component to maintain the proportions of Buffer and acetonitrile and to achieve the appropriate final volume defined for the Diluent. ]
[Note—It is recommended that the Sample solution and Standard solution be prepared concurrently within a narrow window of time (e.g., 30 min) for each drug product sample. ]
Standard solution:
Add 25.0 mL of the Sample stock solution and 15.0 mL of Standard stock solution to a 50-mL volumetric flask, and dilute with Diluent to volume. Pass through a suitable filter of 0.45-µm pore size, discarding the first 3 mL of filtrate.
Sample solution:
Add 25.0 mL of the Sample stock solution to a 50-mL volumetric flask, and dilute with Diluent to volume. Pass through a suitable filter of 0.45-µm pore size, discarding the first 3 mL of filtrate.
CHROMATOGRAPHIC METHOD
Chromatographic system
Mode:
LC
Detector:
UV 300 nm
Column:
4.6-mm × 15-cm; 5-µm packing L85
Column temperature:
30
Flow rate:
1 mL/min
Injection volume:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The typical retention time for 4-aminophenol is about 4.2–5.3 min. ]
Suitability requirements
Resolution:
NLT 1.0 between 4-aminophenol and the nearest peak, Standard solution
Tailing factor:
NMT 1.5 for the 4-aminophenol peak, Standard solution
Relative standard deviation:
NMT 5.0%, Standard solution
Signal-to-noise ratio:
NLT 20 for the 4-aminophenol peak, System suitability solution
ANALYSIS
rU = peak response for 4-aminophenol from the Sample solution
rS = peak response for 4-aminophenol from the Standard solution
WS = amount of USP 4-Aminophenol RS added to the Standard solution (mg)
WU = amount of acetaminophen in the Sample solution (mg)
Samples:
Standard solution and Sample solution
Inject the Sample solution and Standard solution for each drug product sample sequentially, i.e., back-to-back.
Calculate the percentage of 4-aminophenol (C6H7NO) relative to acetaminophen in the portion of drug product taken:
Result = [rU/(rS 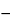 rU)] × (WS/WU) × 100
rU)] × (WS/WU) × 100
rU = peak response for 4-aminophenol from the Sample solution
rS = peak response for 4-aminophenol from the Standard solution
WS = amount of USP 4-Aminophenol RS added to the Standard solution (mg)
WU = amount of acetaminophen in the Sample solution (mg)
Acceptance criteria:
(unless otherwise stated in the monograph): NMT 0.15% of 4-aminophenol relative to acetaminophen
CHROMATOGRAPHIC ADJUSTMENTS
The retention time of 4-aminophenol can be tuned to achieve specificity for a given product matrix. This allowance supersedes provisions in Chromatography  621
621 for adjusting chromatographic conditions and is intended to provide a measure of flexibility when needed. Suggestions for changing 4-aminophenol retention are given in Table 2. The use of a ternary mobile phase system affords ready changes to the ionic strength (water from Solution B) and organic strength (acetonitrile from Solution C), but this can be simplified to a binary mobile phase system.
for adjusting chromatographic conditions and is intended to provide a measure of flexibility when needed. Suggestions for changing 4-aminophenol retention are given in Table 2. The use of a ternary mobile phase system affords ready changes to the ionic strength (water from Solution B) and organic strength (acetonitrile from Solution C), but this can be simplified to a binary mobile phase system.
Table 2
| Condition Change | Change in 4-Aminophenol Retention |
|---|---|
| Increase in organic strength (Solution C) | Decreases 4-aminophenol retention |
| Decrease in pH (Solution A) | Increases 4-aminophenol retention |
| Increase in ionic strength (Solution B) | Decreases 4-aminophenol retention |
| Increase in column temperature | Increases 4-aminophenol retention |
Adjustments to the chromatographic procedure may require verification or validation. See Validation of Compendial Procedures  1225
1225 and Verification of Compendial Procedures
and Verification of Compendial Procedures  1226
1226 for guidance. Adjusted chromatographic conditions must meet all system suitability requirements.
for guidance. Adjusted chromatographic conditions must meet all system suitability requirements.
Solution stability must be verified under actual conditions of use to ensure 4-aminophenol is stable in the Sample solution and the Standard solution as evidenced by NMT ±10% change of the 4-aminophenol peak areas.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Alan R Potts, PhD
Principal Scientific Liaison (301) 816-8364 |
(SM22010) Monographs - Small Molecules 2 |
USP38–NF33 Page 237
Pharmacopeial Forum: Volume No. 39(3)



