The objective of this general chapter is to limit the amounts of elemental contaminants in finished dietary supplement dosage forms labeled as conforming to USP or NF standards. This general chapter is not intended to set limits for dietary ingredients. Those limits are set in the corresponding individual monographs.
The focus of this general chapter is on the four major elements of toxicological concern: arsenic, cadmium, lead, and mercury. The extent of testing can be determined using a risk-based approach that takes into account the likelihood of contamination. Manufacturers should consider the presence of unexpected elemental contaminants to determine compliance.
LIMITS OF ELEMENTAL CONTAMINANTS
The levels of elemental contaminants should be restricted as shown in Table 1 unless otherwise stated in the individual monograph. Specific monographs may provide different limits for articles that need to be consumed in large quantities.
Table 1
| Element | PDE (µg/day)a |
|---|---|
| Arsenic (inorganic) | 15 |
| Cadmium | 5 |
| Lead | 5 |
| Mercury (total) | 15 |
| Methylmercury (as Hg) | 2 |
|
a
Permitted Daily Exposure (PDE) is derived from the Provisional Tolerable Weekly Intake (PTWI) that is recommended by the Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) by subtracting the daily exposure (µg/day) to each elemental contaminant from air, food, and drinking water. A body weight of 50 kg and a safety factor are used to calculate the PDE. Other regulations (e.g., Proposition 65 in California) may require different limits; manufacturers are responsible for compliance with applicable local requirements differing from these PDE values.
|
|
Arsenic may be measured using a nonspeciation procedure under the assumption that all arsenic contained in the supplement is in the inorganic form. Where the limit is exceeded using a nonspeciation procedure, compliance with the limit for inorganic arsenic shall be demonstrated on the basis of a speciation procedure. Methylmercury determination is not necessary when the content for total mercury is less than the limit for methylmercury.
OPTIONS FOR COMPLIANCE WITH THE LIMITS OF ELEMENTAL CONTAMINANTS
In order for a dietary supplement finished dosage form to comply with the limits for elemental contaminants as described in this chapter, the level of elemental contaminant in the finished dietary supplement should be NMT the PDE. The following three options are available for determining compliance with the limits for elemental contaminants in dietary supplements.
Dietary Supplement Analysis Option
This option is generally applicable. In this option the finished dietary supplement dosage form is analyzed according to the procedures in the general chapter Elemental Impurities—Procedures  233
233 or the speciation procedures given in this chapter. The results obtained from the analysis of a typical serving size, scaled to a maximum daily intake, are compared to the PDE, as stated in Table 1.
or the speciation procedures given in this chapter. The results obtained from the analysis of a typical serving size, scaled to a maximum daily intake, are compared to the PDE, as stated in Table 1.
Analysis:
Proceed as directed below in this chapter.
MVSS = measured amount of each elemental contaminant (µg/serving size)
N = maximum daily intake of the supplement recommended in the labeling (servings/day)
Calculate the measured amount of each elemental contaminant, in µg/daily intake, as:
Result = MVSS × N
MVSS = measured amount of each elemental contaminant (µg/serving size)
N = maximum daily intake of the supplement recommended in the labeling (servings/day)
Acceptance criteria:
The measured amount/daily intake is NMT the PDE value given in Table 1.
Individual Component Option
This option is applicable to finished dietary supplement dosage forms with a maximum daily intake of NMT 10 g of the dietary supplement finished product.
Analysis:
Unless otherwise specified in the individual monograph, proceed with the individual ingredient as directed below in this chapter.
Acceptance criteria:
The product meets the requirements when each component used to prepare the finished dietary supplement meets the limits given in Table 2.
[Note—If all components in a formulation meet the limits given for the Individual Component Limits, these components can be used in any proportion. No further calculation is necessary. ]
Table 2
| Element | Individual Component Limits (µg/g)a |
|---|---|
| Arsenic (inorganic)b | 1.5 |
| Cadmium | 0.5 |
| Lead | 0.5 |
| Mercury (total) | 1.5 |
| Methylmercury (as Hg)c | 0.2 |
|
a
The limits for individual components are based on a maximum daily intake of 10 g of a dietary supplement and are intended for use only with Options for Compliance with the Limits of Elemental Contaminants, Individual Component Option.
b
Arsenic may be measured using a nonspeciation procedure under the assumption that all arsenic contained in the supplement is in the inorganic form. Where the limit is exceeded using a nonspeciation procedure, compliance with the limit for inorganic arsenic shall be demonstrated on the basis of a speciation procedure.
c
Methylmercury determination is not necessary when the content for total mercury is less than the limit for methylmercury.
|
|
Summation Option
This option can be used for finished dietary supplement dosage forms that are consumed in quantities greater than 10 g/day or where the acceptance limit for any contaminant in any component of the dietary supplement exceeds the applicable Individual Component Limits.
Analysis:
Unless otherwise specified in the individual monograph, proceed with the individual ingredient as directed below in this chapter.
Calculate the amount of each elemental contaminant, in µg/daily intake, present in the finished dietary supplement dosage form:
Result = S(Ci × Wi) × N
Ci = elemental contaminant concentration in the individual component (µg/g)
Wi = weight of each individual component per serving of the dietary supplement (g/serving)
N = maximum daily intake of the supplement recommended in the labeling (servings/day)
Acceptance criteria:
The calculated amount of each elemental contaminant/daily intake is NMT the PDE value given in Table 1.
ANALYTICAL PROCEDURES FOR TOTAL ELEMENTAL CONTAMINANTS
Performance-based methodology for analysis of total elemental contaminants in general chapter Elemental Impurities—Procedures  233
233 is applicable for dietary supplements. The validation necessity will vary depending on the situation. In all three options described in the section Options for Compliance with the Limits of Elemental Contaminants, the use of Validation of Limit Procedures (see Elemental Impurities—Procedures
is applicable for dietary supplements. The validation necessity will vary depending on the situation. In all three options described in the section Options for Compliance with the Limits of Elemental Contaminants, the use of Validation of Limit Procedures (see Elemental Impurities—Procedures  233
233 ) may be appropriate. However, for the Summation Option, acceptable levels of validation must be determined on a case-by-case basis. Validation of a procedure using the Validation of Quantitative Procedures (see Elemental Impurities—Procedures
) may be appropriate. However, for the Summation Option, acceptable levels of validation must be determined on a case-by-case basis. Validation of a procedure using the Validation of Quantitative Procedures (see Elemental Impurities—Procedures  233
233 ) is acceptable for all options under all circumstances and is generally preferred. The determination of the level of validation necessity is at the discretion of the manufacturer and the competent regulatory authority.
) is acceptable for all options under all circumstances and is generally preferred. The determination of the level of validation necessity is at the discretion of the manufacturer and the competent regulatory authority.
Analytical Procedure for Inorganic Arsenic
Where the level of total arsenic exceeds the limit recommended in this chapter, speciation may be used to determine the amount of inorganic arsenic present. The following procedure is suggested for determination of inorganic arsenic, but any validated procedure shown to give equivalent or better results can be used.
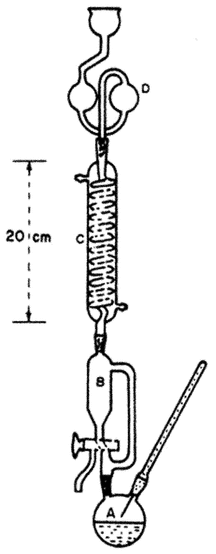
Apparatus
See Figure 1.
Reagents
Distillation-reducing solution:
30.0 g of potassium iodide in 100 mL of water. [Note—Prepare fresh on the day of use. ]
Control:
6.0 µg of arsenic (As) (6.0 mL of Standard Arsenic Solution). [Note—Use this amount rather than the 3.0 mL specified for Standard Preparation in the general chapter Arsenic  211
211 , Method I. ]
, Method I. ]
Sample solution:
Transfer a 2.00-g sample that has previously been ground to pass through a 60-mesh screen to a distillation flask (A). To the flask add 35 mL of hydrochloric acid 6.6 N and 15 mL of Distillation-reducing solution, let stand for 5 min to ensure reduction of arsenic [As(V)], connect the flask to the receiver chamber (B), complete the assembly of the apparatus, and begin circulating tap water through the condenser (C). Half-fill the lower two bulbs of the splash head (D) with water. Maneuver the stopcock to cause the contents of the receiver chamber to drain into the distillation flask, heat the flask until the temperature above the solution reaches 100 –110
–110 , and continue refluxing at this temperature for 15 min. Close the stopcock, continue heating at 108
, and continue refluxing at this temperature for 15 min. Close the stopcock, continue heating at 108 –110
–110 , and collect 30–33 mL of distillate in the receiver chamber. Remove the heating source, and allow the temperature to drop to about 80
, and collect 30–33 mL of distillate in the receiver chamber. Remove the heating source, and allow the temperature to drop to about 80 .
.
Close the stopcock, and add a second 35-mL portion of 6.6 N hydrochloride (HCl) and 15 mL of the Distillation-reducing solution through the thermometer opening to the distillation flask. Replace the thermometer, increase the temperature to 100 –110
–110 , and collect a second 30- to 33-mL portion of distillate in the receiver chamber. Drain the second distillate into the beaker containing the first portion, and cool the combined distillate to room temperature. Remove the splash head, and wash its contents into the beaker. Also, wash down the inside of the condenser and receiver chamber with water, collecting the washings into the beaker. Transfer to a 100-mL volumetric flask, and complete with water to volume.
, and collect a second 30- to 33-mL portion of distillate in the receiver chamber. Drain the second distillate into the beaker containing the first portion, and cool the combined distillate to room temperature. Remove the splash head, and wash its contents into the beaker. Also, wash down the inside of the condenser and receiver chamber with water, collecting the washings into the beaker. Transfer to a 100-mL volumetric flask, and complete with water to volume.
Analysis:
Determine the arsenic content by the ICP-MS procedure in Elemental Impurities—Procedures  233
233 . Alternatively, add 2 mL of potassium iodide TS and 0.5 mL of stronger acid stannous chloride TS to the Sample solution contained in the Erlenmeyer flask, and proceed as directed in Arsenic
. Alternatively, add 2 mL of potassium iodide TS and 0.5 mL of stronger acid stannous chloride TS to the Sample solution contained in the Erlenmeyer flask, and proceed as directed in Arsenic  211
211 , Method I, Procedure, beginning with “Allow to stand at room temperature for 30 minutes.”
, Method I, Procedure, beginning with “Allow to stand at room temperature for 30 minutes.”
Analytical Procedure for Methylmercury
Where methylmercury determination is required, the following procedure is suggested. However, any validated procedure shown to give equivalent or better results can be used.
Procedure 1
This procedure uses an aqueous extraction of the mercury species with an l-cysteine solution, HPLC separation of the derivatized mercury species with a mobile phase also containing l-cysteine, and ICP-MS detection.
Cysteine solution:
1% l-cysteine hydrochloride monohydrate in water
Mobile phase:
0.1% l-cysteine hydrochloride monohydrate and 0.1% l-cysteine free base in water
Standard stock solution:
1 µg/mL of mercury (Hg) as methylmercury chloride, and 1 µg of mercury (Hg) as mercury chloride in 5% hydrochloric acid containing 0.2 mg/mL of l-cysteine hydrochloride monohydrate
Standard solution:
2 ng/mL of mercury as methylmercury from Standard stock solution in Cysteine solution
Sample solutions
For supplements in tablet form:
Weigh, and finely powder a counted number of tablets. Transfer an accurately weighed portion of the powder equivalent to 0.5 times the daily recommended intake to a tared vial. Add 50.0 mL of Cysteine solution accurately weighed, cap the vial, and shake vigorously. Place the vial in a water bath at 60 for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
For supplements in capsule form:
Weigh accurately NLT 20 capsules, and determine the average weight. Place a number of capsules equivalent to about 5 times the daily recommended intake in a blender, add 500.0 mL of Cysteine solution and blend to obtain a homogenous analytical suspension. Transfer 50.0 mL of this analytical suspension to a vial, cap the vial, and shake vigorously. Place the vial in a water bath at 60 for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
For supplements in liquid form:
Weigh accurately an amount of the liquid equivalent to 0.5 times the daily recommended intake into a vial, and add 50.0 mL of Cysteine solution. Cap the vial, and shake vigorously. Place the vial in a water bath at 60 for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
for 60 min. Shake the vial again, and return to the water bath for another period of 60 min. Shake the vial again, and allow to cool at room temperature for about 20 min. Filter through a 0.45-µm polyethylene membrane. [Note—Prepare fresh on the day of use. ]
Chromatographic system
Mode:
LC
Detector:
ICP-MS at a mass-to-charge ratio of 202
Column:
4.6-mm × 15-cm; 4-µm packing L1
Flow rate:
1 mL/min
Injection volume:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 3 between the peak representing mercury (Hg2+) species at a relative retention time of about 0.56 and the peak representing methylmercury (MeHg+) species at a relative retention time of 1.0
Relative standard deviation:
NMT 10.0%, Standard solution
Analysis
Samples:
Standard solution and the appropriate Sample solution
Determine the peak area of the peak representative of methylmercury in the chromatograms of the Sample solution and Standard solution.
Acceptance criteria:
0.2 µg/daily intake: The peak area for methylmercury in the chromatogram of the Sample solution is NMT the peak area for methylmercury in the chromatogram of the Standard solution.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Christopher O Okunji, Ph.D.
Scientific Liaison, Dietary Supplements (301) 230-3244 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 1783
Pharmacopeial Forum: Volume No. 39(5)