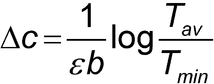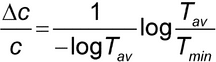Add the following:
THEORY
Ultraviolet-visible (UV-Vis) spectroscopy is an electronic transition spectroscopic technique in which the interaction between incident radiation and electrons results in the promotion of one or more of the outer or the bonding electrons from a ground state into a higher-energy state. This quantum effect results in a specific absorption of radiation, the frequency and wavelength of which are governed by the equation:
E = h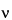 = (hc/
= (hc/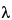 ) × 109
) × 109
where:
E = energy
h = Planck's constant (6.63 × 10-34J·s)
c = velocity of light (2.998 × 108 ms-1)
Even the simplest molecules have a large number of discrete energy levels and closely spaced levels adjacent to them caused by atomic vibration within the molecule. Overlap of these vibrational bands onto the electronic spectrum causes the measured spectra to appear as a broad, bell-shaped peak. As a general rule, most molecules absorb somewhere in the UV-Vis region. The greater the extent to which the p electrons are delocalized, the longer the wavelength of the first absorption band, i.e., the band of lowest energy and longest wavelength.
Derivative Spectroscopy
The advantages of derivative spectroscopy as an analytical tool have been known since the 1950s. Before the availability of the personal computer, generating derivative spectra electronically was complex and difficult, and for this reason the technique was rarely used. The introduction of microcomputers in the late 1970s simplified the generation of digital spectra and the associated mathematical manipulations required to produce first- and higher-order derivatives. This significantly increased the use of the derivative technique.
Derivative spectroscopy uses first or higher derivatives of absorbance (A) with respect to wavelength for qualitative analysis and for quantitation where:
A = f(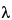 ) Zero order
) Zero order
A first-order derivative is the rate of change of absorbance with respect to wavelength (Figure 1). A first-order derivative starts and finishes at zero. It also passes through zero at the same wavelength as 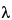 max of the absorbance band. On either side of this point are positive and negative bands with a maximum and minimum at the same wavelengths as the inflection points in the absorbance band. This bipolar function is characteristic of all odd-order derivatives.
max of the absorbance band. On either side of this point are positive and negative bands with a maximum and minimum at the same wavelengths as the inflection points in the absorbance band. This bipolar function is characteristic of all odd-order derivatives.
First-order derivatives are used principally for background absorption minimization or elimination for measurements on turbid, scattering solutions and suspensions and for analysis of trace components in complex absorbing matrices.
The most characteristic feature of a second-order derivative is a negative band with minimum at the same wavelength as the maximum on the zero-order band. A second-order derivative also shows two additional positive satellite bands on either side of the main band. A fourth-order derivative shows a positive band. A strong negative or positive band with a minimum or maximum at the same wavelength as 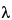 max of the absorbance band is characteristic of the even-order derivatives.
max of the absorbance band is characteristic of the even-order derivatives.
Higher-order (second and higher) derivatives can be used to:
-
enhance resolution of overlapping peaks for the separation of superimposed spectra—particularly useful in multicomponent analysis
-
assist quantitative determination of trace components
-
aid characterization of individual pure compounds, particularly for archiving purposes and for complementing the information obtained from other techniques such as infrared, nuclear magnetic resonance, and mass spectroscopy
-
assist in purity testing of products
Note that the minimum number of bands observed is equal to the derivative order plus one.
INSTRUMENTATION
All modern UV-Vis measurements involve detecting and measuring the intensity ratio of the radiation at a certain wavelength in the presence or absence of the absorbing sample. Figure 2 is a schematic of a double-beam spectrophotometer. Dispersion of light to achieve the desired resolution can occur before or after introduction of the sample, but all commercial UV-Vis instruments share the following features to perform these functions:
-
continuum source
-
monochromator or polychromator
-
sampling area
-
detector
Care must be taken to ensure that the sample is not degraded by the incident light beam and that any heating effects are minimized. This is particularly important when diode-array instruments are used because the sample is irradiated at all wavelengths.
Continuum Source
Two major types of continuum source are currently in use: continuous and pulsed. Continuous sources include tungsten halogen for visible, deuterium arc for UV, and xenon arc for both. The source for pulsed radiation is the xenon flash lamp. Many UV-Vis instrument systems use a combination of deuterium and tungsten halogen to effectively cover the UV and visible regions, respectively. By necessity, source selection is achieved either by the use of a mirror or by physical movement of the lamps. This change usually is performed in the region of 320–350 nm, and thus qualification of the system must be performed using both source and mirror positions. Systems based on xenon lamps have the benefit of a single source and a higher energy output, but they are more expensive.
Monochromator
The wavelength scale can be encoded by either a scanning monochromator or a grating polychromator (see Figure 3), as is the case for spectrophotometers equipped with linear or two-dimensional array detectors. A discussion of the specific benefits and drawbacks of each of the dispersive designs is beyond the scope of this general chapter. Any properly qualified instrument should be suitable for qualitative measurements. Care must be taken when selecting an instrument for quantitative measurements because dispersion, response linearity, and stray light may not be uniform across the full spectral range.
Sampling Area
Numerous sampling arrangements are available in addition to the cell holders that are designed to accommodate various path-length configurations based on conventional rectangular cells. These include flow cells, fiber-optic-based immersion probes, micro-well plate configurations, and automated sample changers, among others. Considerations such as sampling volume, speed of measurement, and reproducibility of sample presentation should be evaluated to optimize the sampling device for specific applications.
Detector
Photoelectric detectors, which are the most common form of UV-Vis detectors, generate an electric current that is directly proportional to the intensity of the radiant energy incident upon them. They may take the form of photosensitive semiconductor devices, either discrete detectors, linear or two-dimensional arrays, or photomultipliers. Photosensitive semiconductor devices include solid-state photodiodes, charge-coupled device arrays (CCDs), and phototransistors. The most common semiconductor material is silicon, which is sensitive to wavelengths of 400–800 nm, but some silicon devices have extended sensitivity from as short a wavelength as 190 nm to as long a wavelength as 1100 nm. The dynamic response of these detectors typically is four orders of magnitude. Most array detectors are made of silicon and hence have a similar wavelength response. Other semiconductor materials can provide wavelength response to several micrometers.
In contrast, photomultipliers are vacuum detectors that have a photocathode in which photon energy releases electrons that are directed by the field applied to electron-sensitive plates. By a cascade effect, these dynodes amplify the electrons released initially by the absorption of the incident radiation. Photomultipliers have typical wavelength responses of 160–900 nm, although some photocathode materials can provide response to higher wavelengths. The dynamic response of these detectors typically is six orders of magnitude or higher.
Alternative UV-Vis Detector Configurations
diode-array instruments
In a diode array, the optical configuration is reversed from that in a conventional spectrophotometer, and the light beam passes through the sample before being dispersed by the polychromator. This gives the benefit of fast, full spectral data with no moving parts that can wear out. The perception is therefore that diode arrays are more reliable than other detectors. This is true, but the reverse-optics design requires that both the sample and optics before the dispersing element (usually a grating) are subjected to the full-spectrum radiation also encountered by these components in a conventional spectrophotometer. In a conventional spectrophotometer, the sample and optics are outside the monochromator, and optical beam deflection and/or scattering simply reduces the intensity of the light that enters the monochromator. In an array-based spectrophotometer, deflection occurs within the monochromator, causing scatter within the confined environment and an associated increase in stray light that leads to a reduction in optimum photometric range.
hplc detectors
The design of a UV-Vis spectrophotometer is always the result of a number of considered compromises. For example, optical performance (absolute wavelength resolution) is governed by the focal length of the monochromator, which in turn dictates the physical size of the instrument. In the case of HPLC detectors the need to provide a high-stability, low signal-to-noise ratio output at high transmittance levels through a small-aperture flow cell requires that by design these systems may not have the dynamic range or wavelength accuracy of their conventional spectroscopic counterparts.
fiber-optic-based modular systems
In recent years there has been a rapid increase in the availability and use of systems built around the ability of fiber optics to channel and multiplex optical systems. Although these systems have the advantages of flexibility and ease of use and they allow measurements to be performed on micro-plates, customized systems, etc., analysts must consider the following disadvantages:
-
Some analysts assume that these array-based fiber-optic systems are immune to room light interference at the sample interface, which may or may not be true. This assumption is easily tested by using a black photographic film-changing bag or a simple black cloth. Use of a masking technique should not change the measured value if the assumption is true.
-
Custom-built systems do not have additional shuttering, stray light filtering, and other capabilities that are found in commercially designed spectrophotometers, and thus their performance characteristics, i.e., the optimum photometric range, may be significantly reduced.
-
Light levels transmitted directly down fibers from high-intensity sources such as Xenon flash lamps may cause photodegradation. In simple self-built systems, the source may be coupled by fiber directly to the sample interface, and the only control is the source on/off switch.
CALIBRATION
UV-Vis instrument calibration involves two components: primary wavelength (x-axis) and intensity (y-axis), and often is performed when the instrument is initialized. Most dispersive UV-Vis instruments use atomic emission lines from either the source or a secondary lamp for primary wavelength (x-axis) calibration, in addition to the zero-order position in the monochromator. Depending on the vendor's configuration of the instrument, these calibration procedures may be available to the user. Calibration of the photometric scale (y-axis) is critical for successful quantitation and method transfer between instruments. Although the fundamental measurement relies on a simple ratio measurement, modern instruments may use several gain settings or amplification factors to ensure a linear detector response, particularly at low intensity levels and high power settings. These gain settings often are initially set by the manufacturer, but the settings may be recalibrated as the optics age or as other changes take place.
Detailed functional validation based on certified reference materials is recommended to demonstrate the suitability of laboratory instruments, even for instruments that possess an internal calibration capability. The use of external reference materials does not obviate the need for internal quality control procedures. Rather, it provides independent documentation of the fitness of the instrument to perform the specific analysis.
ANALYTICAL CONSIDERATIONS
Instrumental Factors
spectral bandwidth
Because most spectrophotometric procedures require good spectral resolution, the spectral bandwidth is of great importance. Analysts should use the narrowest slit width that will provide an adequate signal-to-noise ratio because optimum resolution is achieved when the signal-to-noise ratio is maximized. If access to a variable-bandwidth instrument is available, then the optimum setting can be defined as the largest bandwidth at which no significant reduction in peak intensity is observed. In practical terms for molecules of pharmaceutical interest in solution, a spectral bandwidth of 2 nm is considered adequate.
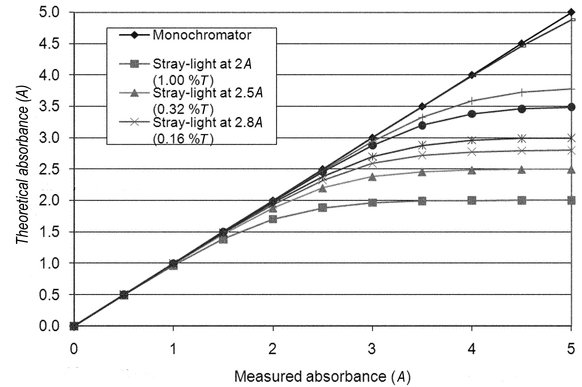
stray light
Stray radiation, commonly referred to as stray light, can be defined as radiant energy at wavelengths other than those indicated by the monochromator setting and all radiant energy that reaches the detector without having passed through the sample or reference solutions (see Figure 4). It may be caused by any scattered radiation from imperfections in the dispersing medium, which commonly is a grating. The use of a holographic grating substantially reduces the levels of this source of stray radiation. Higher-quality ruled gratings yield a low level of scattered radiation. In higher-performance instruments, stray radiation can be reduced by the use of double monochromators or double-pass monochromators. Stray radiation, or apparent stray radiation, also may be caused by light leaks in the system, incorrect wavelength calibration, incorrect optical alignment, reduced source output, or reduced detector response.
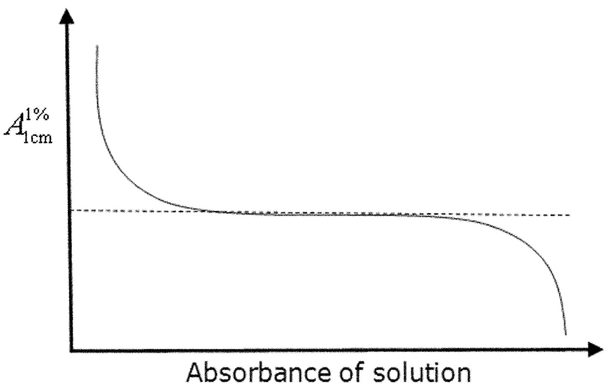
optimum working photometric range
Determination of the optimum photometric range is fundamental to establishing the capability of a given instrument before and during method validation. The importance of this procedure can be shown by the fact that in 1945 Vandenbelt et al. (1) attempted to establish the optimum absorbance range on the then-new Beckman DU spectrophotometer. They suggested a simple approach in which the molar absorptivities of various compounds are measured at different concentrations. This approach is shown theoretically in Figure 5, where the center plateau is used to define the photometric range.
In practice, data are more variable. Figure 6 shows three typical curves.
Figure 6. Molar absorptivity vs. absorbance for 3 compounds: (a) aqueous potassium nitrate measured at 301 nm, (b) potassium chromate in 50 mM potassium hydroxide at 373 nm, and (c) potassium chromate in 50 mM potassium hydroxide at 273 nm. Redrawn with permission from (2).
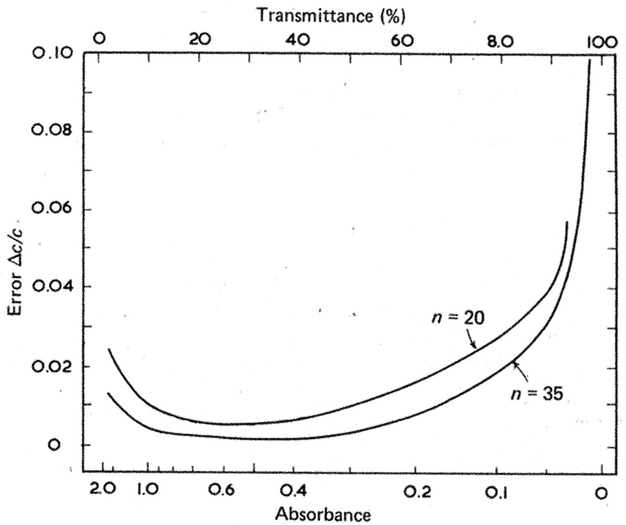
A more rigorous method for estimating an instrument's region of maximum precision is given by Youmans and Brown (3). The error in the measurement of the concentration of a solution of transmission T can be expressed as:
where Dc is the change in concentration,  is the molar absorptivity of the compound, b is the path length, and Tav and Tmin are the mean and minimum values, respectively, from a series of n measurements on the same sample.
is the molar absorptivity of the compound, b is the path length, and Tav and Tmin are the mean and minimum values, respectively, from a series of n measurements on the same sample.
The statistical error is (see Figure 7):
The noise levels, and hence the precision of measurement, depend primarily on detector noise type, as shown in Figure 8.
Figure 8. Measurement precision as a function of noise type. Calculated and redrawn with permission from (4).
Both approaches clearly show that in addition to the expected increasing error at higher absorbance levels caused by stray light and other factors, there is a similar, if not larger possibility for increasing error at low absorbance levels caused by instrumental variances in the form of noise from the detector, source, etc. This variance often is overlooked in the desire for economy of scale, when analysts use smaller samples and shorter path lengths and therefore lower measured absorbance values.
Sample-Based Factors
The most important sample-based factors that negatively affect quantitative UV-Vis spectrophotometry are fluorescence and light scattering. If the sample matrix includes fluorescent compounds, the measured signal usually will contain a contribution from fluorescence. The wavelength range and intensity of the fluorescence depend on the chemical composition of the fluorescent material. Suspended particles scatter light by the Tyndall effect, causing a decrease in the measured intensity that increases as the wavelength decreases. Unless there is no alternative, absorbance should not be determined on turbid samples. Procedures for removing turbidity include filtering, centrifuging, or flocculating the sample and are performed before any additional procedures that generate a chromophore, provided that they do not affect the concentration of the analyte or the chromophore in the test solution. Any measurements performed on a turbid solution are highly instrument specific and can be used only for comparative purposes in the same system. A further complication can arise at higher analyte concentrations with respect to the coordination chemistry and system matrix. Ionic association, complex formation, and similar factors can cause deviation from the expected linear response.
Sampling Factors
cells
Quantitative absorbance measurements usually are made on solutions of the substance in liquid-holding cells. The most common cell path length is 10 mm, although path lengths from 0.01 to 100 mm are commercially available (see Figure 9). For samples with low absorbance, improved sensitivity generally can be obtained by increasing the cell path length. For example, the theoretical absorbance of a solution in a 50-mm cell is greater by a factor of five compared to the same solution in a 10-mm cell. Errors in absorption readings arising from cells almost always are caused by dirty windows that can absorb a significant proportion of the incident light beam. Less frequent causes of error are an incorrect choice of cell material for the wavelength required, e.g., use of glass cells less than 320 nm, nonrepeatability of cell positioning, differences in cell window thickness, nonparallel optical windows, or impurities in cell window materials. A 10-mm cell manufactured with a ± 0.05-mm tolerance will add a 0.005 A contribution to the overall uncertainty, when measuring absorbance values at the 1-A level. Ten-mm cells with a ± 0.01-mm tolerance are commercially available. As a general guide, the cell chosen should have a transmittance value of > 75% when filled with the appropriate solvent and measured at the wavelength of interest.
Analysts can mitigate the variability introduced by cells by adhering to the following set of best practices. Carefully clean cells before use, and store them in a manner that avoids contamination when they are not in use. Cells in frequent and repeated use, e.g., flow cells, can be stored wet in high-purity water or 1% (v/v) nitric acid. Do not use cracked or scratched cells. Wipe cells carefully with a soft, clean, lint-free cloth before they are placed in the spectrophotometer. Do not use lens-cleaning paper or any other form of paper cleaning tissue because these will either scratch or allow contamination of the optical face.
It is preferable to use a single cell for all measurements of both the reference and samples. When several sample cells must be tested against one reference cell, they all should be closely matched in their characteristics. Whenever possible, measure standards and samples at the same time and under the same conditions to minimize the potential for bias. The reference cell should contain the same solvent as the sample cell and should be checked against the sample cell at all wavelengths at which measurements are required. Analysts can apply corrections for differences between cells to absorbance readings obtained from the solutions under examination. The maximum allowable correction is ± 0.01 absorbance unit. Best practices include using the same cell in the reference beam with the same face incident to the light beam, and flushing the sample cell three times with the sample solution before the final filling before measurement. The use of flow cells effectively addresses many of these cell-handling and cell-filling issues. The contents in both the sample cell and the reference cell must be free from gas bubbles and particles. If flow cells are used, analysts may be required to degas the solution(s) before measurement in order to avoid the formation of air bubbles in the cell.
A sequence of activities is used to monitor the quality of the measurement process and to ensure that the contents are representative of the sample under measurement. Drift of the spectrophotometric baseline can be detected by measuring the blank at the start and end of the sample sequence. Adequate flushing of the cell can be confirmed by alternating between measurement of the standard (high concentration) and the blank (zero concentration) at the start of the measurement sequence. The latter check is particularly important for automated systems that can generate reproducible but inaccurate results because of inadequate flushing.
care of cells
Contaminated cells are the greatest single source of error in spectrophotometry. Cells should never be handled by the optical polished faces, and analysts should rinse off residual or spilled solution. If cells are properly cared for, rigorous cleaning methods rarely will be needed, especially if the cells are cleaned immediately after use. Although acid solutions will simply contaminate surfaces, alkaline solutions can etch all types of polished glass surfaces, and the degree of attack depends on the pH and contact time. When cleaning cells, analysts should ensure that the optical faces are not scratched or chipped. Commercial cleansing solutions are available and can be used, provided that they are diluted before use in accordance with the manufacturers' recommendations. After they are cleaned with such agents, the cells should be carefully and thoroughly rinsed with high-purity water. If this fails to clean the cells, then soaking them in cold, concentrated nitric or hydrochloric acid is acceptable. Flow cells are best cleaned in situ, provided that the solvent does not interact with the connecting tubing. If the cells are stored for an extended period, then they should be dried quickly after cleaning by blowing dry with a compressed gas stream in a clean, dust-free environment.
alignment and filling of cells
Analysts should align the cells so that the same optical face is always incident to the light beam. Most cells have type identification engraved on one face: this can be used to ensure consistent orientation. If there are no markings, a small mark can be made on one face outside the area of the light beam. The use of cells with no type markings is not recommended, but if they are used analysts should confirm their suitability for the intended application. If cells are provided with the instrument, individual cell holders should always be used in the same beam as identified, e.g., by use of appropriate markings.
cell corrections
If multiple cells are used, differences in the optical transparency of individual cells can introduce a systematic bias unless the latter is accounted for by a cell correction. Do not use corrections in excess of 0.01 absorbance unit. To determine if cell corrections are necessary, analysts can fill all cells with the appropriate solvent and measure the differences in absorbance at the required analytical wavelengths. These measurements are repeated after cleaning to ensure that differences in absorbance are related to the cells and are not the result of contamination. Analysts should fill and check the cells repeatedly until the results are consistent, and they should investigate any appreciable change in cell correction because it indicates contamination, damage to the cells, or incorrect adjustment of the instrument.
OTHER SOURCES OF INFORMATION
-
Vandenbelt, J.M., Forsyth, J. and Garrett, A. (1945) Anal. Chem. (Industrial and Engineering Chemistry, Analytical Edition), 17(4), 1945, 235
-
Standards and Best Practice in Absorption Spectrometry, Ed. C. Burgess & T. Frost, Blackwell Science, (1999), ISBN 0-632-05313-5.
-
Youmans, H.L. and Brown, W.D. (1976) Anal. Chem., 48, 1152.
-
Skoog, West & Holler (7th Ed) 1996, Table 24-4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 1733
Pharmacopeial Forum: Volume No. 37(5)