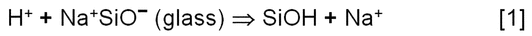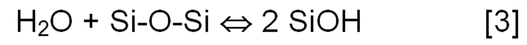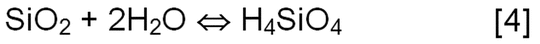PURPOSE
This general information chapter provides information about factors that affect the durability of the inner surface of glass containers. Recommended approaches are provided to evaluate the potential of a drug product to cause formation of glass particles and delamination of the inner surface. Screening methods are provided to detect glass particles and delamination, allowing a comparison to be made of glass durability on a lot-to-lot basis or between different glass manufacturers.
SCOPE
This chapter addresses bottles and vials manufactured by molding and ampuls, cartridges, vials, and prefillable syringes manufactured from tubing glass. Glass for pharmaceutical packaging is classified as Type I borosilicate glass, Type II treated soda-lime-silica glass, or Type III soda-lime-silica glass on the basis of the hydrolytic resistance of the glass, as defined in Containers—Glass  660
660 . Type I glass containers are suitable for most products for parenteral and nonparenteral use. Type II glass containers are suitable for most acidic and neutral aqueous products for parenteral and nonparenteral uses, and can be used for alkaline parenteral products when stability data demonstrate their suitability. Type III glass containers usually are not used for parenteral products or for powders for parenteral use, except when suitable stability test data indicate that Type III glass is satisfactory. This chapter focuses primarily on Type I glass, because it is the most widely used in the pharmaceutical and biopharmaceutical industry for parenteral products although the guidance can be equally applied to Type II and Type III glass used for parenteral products.
. Type I glass containers are suitable for most products for parenteral and nonparenteral use. Type II glass containers are suitable for most acidic and neutral aqueous products for parenteral and nonparenteral uses, and can be used for alkaline parenteral products when stability data demonstrate their suitability. Type III glass containers usually are not used for parenteral products or for powders for parenteral use, except when suitable stability test data indicate that Type III glass is satisfactory. This chapter focuses primarily on Type I glass, because it is the most widely used in the pharmaceutical and biopharmaceutical industry for parenteral products although the guidance can be equally applied to Type II and Type III glass used for parenteral products.
The chapter should be useful for the following:
- Molded and tubular glass container manufacturers and converters
- Pharmaceutical and biopharmaceutical companies
- Contract manufacturing and filling organizations
Glass delamination may be described as the appearance of thin flexible flakes of glass (or lamellae) that can range in size from <50 µm to 200 µm in a drug product solution. This is a serious quality issue and can result in a product recall. The appearance of glass lamellae is a lagging indicator of a strong interaction between the drug product and the inner surface of the glass. Although delamination is the most obvious visual indicator, it represents the final stage of a complex glass corrosion reaction, and can be observed only at a point where prevention is no longer an option. Adding further complexity to detection, mechanical energy from shaking or vial-to-vial contact during transportation may be required to dislodge the lamellae from the internal surface of a filled vial and facilitate observation.
Tests for delamination combine the visual examination of the solution, an examination of the vial's internal surface and analysis of an aggressive test solution to assess the propensity of the internal glass surface of vials to delaminate. These examinations and the use of an aggressive test solution are intended to be conducted by the pharmaceutical manufacturer, not the glass manufacturer or converter.
GLASS TYPES
Glass in its pure form consists of silicon dioxide with a melting point in excess of 1700 . However, this is rarely used commercially because of the cost of working at these elevated temperatures. Added network modifiers, such as sodium, potassium, or boron oxide, lower the melting point and lower the chemical durability, whereas added network stabilizers, such as calcium and aluminum oxides, improve the durability of the glass. Colored glass (e.g., amber glass) is produced by transition metal oxides such as iron oxides. All additives to pure silicon dioxide, as well as silicon itself, can be viewed as potential extractables from glass containers.
. However, this is rarely used commercially because of the cost of working at these elevated temperatures. Added network modifiers, such as sodium, potassium, or boron oxide, lower the melting point and lower the chemical durability, whereas added network stabilizers, such as calcium and aluminum oxides, improve the durability of the glass. Colored glass (e.g., amber glass) is produced by transition metal oxides such as iron oxides. All additives to pure silicon dioxide, as well as silicon itself, can be viewed as potential extractables from glass containers.
Glass compositions do not exist as stoichiometric chemical compounds but rather are expressed by a range of compositions. Thus, there is allowable variation within a glass type, and glass types may vary slightly among glass producers. Soda-lime-silica glass consists of silicon dioxide (60–75 wt%), sodium and potassium oxides (12–18 wt%), and smaller amounts of calcium, magnesium, and aluminum oxides (5–12 wt%). This glass has a relatively high coefficient expansion (COE) of 80–90 × 10 7 per degree and is susceptible to breakage by thermic shock. Borosilicate glass consists of silica (65–80 wt%), boric oxide (7–13 wt%), and smaller amounts of sodium, potassium, and aluminum oxides. The presence of boron provides greater resistance to thermal shock through a reduction in COE and to hydrolytic attack by increasing the connectivity of the glass network. Type I glass is available in multiple formulations: tubular glass is available with a low COE, described as 32–33 expansion glass and with a relatively low COE (range, 48–56 expansion), for example 51 expansion glass, in reference to their individual COEs of 32.5 × 10
7 per degree and is susceptible to breakage by thermic shock. Borosilicate glass consists of silica (65–80 wt%), boric oxide (7–13 wt%), and smaller amounts of sodium, potassium, and aluminum oxides. The presence of boron provides greater resistance to thermal shock through a reduction in COE and to hydrolytic attack by increasing the connectivity of the glass network. Type I glass is available in multiple formulations: tubular glass is available with a low COE, described as 32–33 expansion glass and with a relatively low COE (range, 48–56 expansion), for example 51 expansion glass, in reference to their individual COEs of 32.5 × 10 7 per degree and 51.0 × 10
7 per degree and 51.0 × 10 7 per degree, respectively. Molded glass has a higher COE in the region of 60–63 expansion.
7 per degree, respectively. Molded glass has a higher COE in the region of 60–63 expansion.
FORMATION OF MOLDED AND TUBULAR GLASS CONTAINERS
Formation of molded and tubular glass containers requires a number of steps. The quality of the container used in packaging depends on the conditions and the quality control of each step. Both molded and tubular containers originate from a glass furnace, and different furnaces are dedicated to borosilicate or soda-lime-silica glass. The refractory bricks lining the furnace deteriorate with time and must be replaced. Worn bricks can contribute to cosmetic defects such as stones (inclusions in the glass) that become incorporated into the molded glass containers or glass tubing.
Molded glass vials and bottles are manufactured in a one-step process whereby a stream of molten glass is cut into a gob, which then enters a mold where air or tooling is used to shape the container to the mold. Formation of containers from tubing glass is a two-step process. Glass tubes of a specific diameter are formed from a stream of molten glass that exits the furnace, is cooled, and is sectioned into standard lengths. These tubes are subsequently converted into glass containers (ampuls, cartridges, syringes, or vials) by either the tubing glass manufacturer or by independent converters. It is technically difficult to form glass tubing with a diameter sufficient to make bottles containing 100 mL or more, so these containers are produced by molding.
Gas flames are used to soften tubing glass to form the neck, to melt the glass to form the base of ampuls or vials, and to separate the container from the glass tube. In the case of cartridges and prefillable syringes, the glass tube is cut to length, and the ends are softened to form the nozzle and flange of the syringe and the neck and rear of the cartridge. Heating rate, maximum glass temperature, and production speed are critical parameters that can be adjusted for individual forming machines. After formation, both tubular and molded containers pass through an annealing oven (lehr) that heats the containers to 20 to 30
to 30 above the transformation temperature (Tg) of the individual glass formulation (Tg for borosilicate glass is approximately 570
above the transformation temperature (Tg) of the individual glass formulation (Tg for borosilicate glass is approximately 570 ) and then gradually cools them in order to remove stresses in the container due to the manufacturing process. This too is a critical process because poorly annealed containers show reduced chemical and mechanical durability.
) and then gradually cools them in order to remove stresses in the container due to the manufacturing process. This too is a critical process because poorly annealed containers show reduced chemical and mechanical durability.
The process of forming tubular vials and ampuls has an effect on the local surface composition of the glass. During formation of the neck and particularly the base, the temperature of the inner surface of the containers can exceed the evaporation point of some of the glass components such as alkali borates. Under certain time–temperature conditions, the glass can phase separate during forming, creating nonhomogenous surface chemistry on the interior of the container. Both scenarios are undesirable for the storage of aggressive liquids from a surface chemical durability perspective. Evidence of this can be obtained by appropriately etching the glass with acid, after which an opaque ring will appear above the heel of the container, indicating a negative change in the inner surface chemistry. The same phenomenon can be observed at the shoulder of the container as well, but in many instances this area does not experience prolonged contact with a liquid.
PROCESSING OF MOLDED AND TUBULAR GLASS CONTAINERS
At times, the inner surfaces of glass ampuls, vials, and bottles undergo additional treatments. As an example, heating glass propagates sodium oxide toward the inner surface of the container, but washing with water does not remove sodium oxide because of the latter’s limited solubility. When glass is exposed to an aqueous solution, sodium ions diffuse into the solution from the glass surface to produce hydroxide ions, resulting in an elevated pH in unbuffered solutions. One common treatment is the use of ammonium sulfate which converts the sodium oxide on the inner surface to a depth of approximately 10–100 nm into highly soluble sodium sulfate that then can be removed by washing. Although removal of sodium ions from the surface does reduce the propensity for pH shift, the treatment does remove structural elements, leaving a thin silica-rich inner surface layer. The process originally was designed to raise the surface hydrolytic resistance of Type III soda-lime-silica glass to that of Type II glass in order to mimic the hydrolytic resistance of Type I glass. This process also can be applied to Type I glass.
In summary, the key factors that influence glass surface durability of containers manufactured from Type I glass are primarily the manufacturing conditions, such as the forming temperature, the time of exposure to heat, and the annealing conditions. The temperatures used for subsequent steps are lower than those used for forming and annealing (see Table 1), and do not pose an additional risk to the chemical durability of the glass from phase separation or volatilization. Post-manufacturing operations such as storage in humid conditions and processing, such as depyrogenation in the presence of water vapor and terminal sterilization via autoclaving, can also impact glass surface chemical durability.
Table 1. Temperatures Encountered During Formation and Processing of Type I Tubular Glass Containers
| Key Operations |
Typical Temperatures ( |
|---|---|
| Furnace | 1500–1650 |
| Sectioning of tube and base formation | 1300–1500 |
| Working range | 1000–1250 |
| Softening | 750–850 |
| Annealing | 550–600 |
| Depyrogenation range | 250–350 |
| Terminal sterilization | 110–130 |
GLASS CONTAINER SOURCING
A pharmaceutical manufacturer has a range of choices when selecting a glass container for a drug product. These include the type of glass (I, II, or III), the production method (tubular or molded), surface treatments, as well as the size and neck finish of the container. It is important that the pharmaceutical manufacturers provide sufficient information on their requirements, such as the drug product formulation and the manufacturing and filling process, to allow the glass vendors to make informed judgments as to what containers to recommend.
Pharmaceutical manufacturers should consider the upstream provenance of the containers they purchase in that they should have sufficient knowledge of the glass manufacturing process and glass composition. This is essential to qualify a particular glass container type from a glass manufacturer for a particular drug product. The following knowledge is useful in this regard:
- Glass formulation
- COE for Type I tubular glass (32–33 or 48–56 expansion)
- Whether the glass converter makes its own glass feedstock or sources the glass feedstock from a third party
- The manufacturing site for the glass containers. If multiple sites manufacture glass containers for a given product, information to determine if the glass containers made at the different sites will perform comparably
- Whether the glass surface has been modified through chemical treatment such as ammonium sulfate by the glass manufacturer or converter.
The maker and user of a glass container should collaborate to assure that glass quality is monitored and maintained throughout the extent of the glass supply relationship. Glass quality should also be monitored and inferred by the user through observations made during storage throughout the product’s use-by-date. The glass manufacturer and glass user quality management programs should include the following:
- Quality audits of glass supplier (glass manufacturer and/or converter) by the glass user
- Establishing mutually agreed upon acceptable quality levels for lots of glass containers
-
Monitoring and trending of the quality of glass batches, including but not necessarily limited to, monitoring the values obtained by the Surface Glass Test in
 660
660
- Monitoring and trending of glass quality and glass manufacturing process performance by the glass container manufacturer, including the effectiveness of methods used during the manufacturing process of the glass container to measure geometric tolerances and identify cosmetic defects
- Assurance that differences among different glass manufacturing sites do not significantly affect quality of a specified glass container sourced from multiple sites
- Presence of a system to monitor and qualify changes made to the glass manufacturing process and to inform customers of such changes.
GLASS SURFACE CHEMISTRY
After manufacturers are assured of the quality and consistency of the glass containers they purchase, they can use the complex aqueous chemistry of surface glass to decide on potential drug product formulation and treatment steps that could increase glass stability. The first reaction between the glass surface and an aqueous phase (water or water vapor) involves ion exchange between hydrogen ions (or hydronium ions H3O+) from the aqueous phase and alkaline ions in the glass (Equation 1). This ion-exchange occurs in a short reaction time in acidic or neutral solutions. In basic solutions, the reaction occurs at the glass/water interface and dissolves the silica network. Further reaction of the silica releases silicic acid into the solution, thereby lowering the pH (Equation 2). These reactions result in hydration of the glass surface and an alkali-depleted, silica-rich layer.
The presence of water in the leachate promotes hydrolysis of the Si–O bond forming a silica-gel layer (Equation 3).
The mechanical properties of the surface gel that forms are different from those of bulk glass. Repeated hydration and dehydration of the layer leads to the cracking of the gel layer and eventual generation of particles. This process is worsened as the gel layer increases in thickness. This phenomenon is well known in glass exposed to ambient moisture (known as weathering). At higher pH values, the mechanism of glass degradation changes from the leaching of alkali elements to the dissolution of the silicate network as shown in Equations 4 and 5.
Reaction (Equation 5) increases the solubility of the silicic acid in solution, driving the reaction forward. At some point the limit of solubility is exceeded, and particles are formed via precipitation. If the solution is not buffered, a decrease in the solution pH will take place. These reactions and scenarios apply only to the reactions of glass with water; the presence of drug product formulations can complicate the situation considerably.
FACTORS THAT INFLUENCE INNER SURFACE DURABILITY
A number of factors have the potential to negatively influence the chemical durability of the inner surface of glass containers. These factors include glass composition, the conditions under which the containers were formed, subsequent handling and treatments, and the drug product in the container (Table 2). Not only can an aggressive drug substance corrode the inner surface, but excipients such as buffers, chelating agents and organic acids and high pH can also have a deleterious effect. For example, neutral solutions of sodium citrate attack glass with a severity similar to that of substantially alkaline solutions. Organic acids, such as gluconic and malonic acids, also corrode glass through a proposed mechanism of an ion exchange reaction in which metal ions on the glass surface are replaced by hydrogen ions from the acid. Not all listed factors negatively influence surface durability to the same degree, and can contribute to delamination either acting alone or in combination. Because of the range of variables, end users should examine all relevant variables for an individual drug product and assess the degree of risk for delamination and formation of subvisible and visible glass particles. In some situations, the accumulation of risk factors may indicate that the selection of a glass container for a particular formulation should be done following a predictive screening study to establish more stringent glass quality requirements or may indicate that a glass container should not be used for a formulation.
Table 2. Factors That Influence the Inner Surface Durability of Glass
| Container Manufacture | Container Processing and Storage |
Drug Product: Formulation, Processing, and Storage |
|---|---|---|
| • Glass composition • Molded or tubular container • Tubular manufacturing process: — Converting speed — Converting temperature |
• Post-formation treatments: — Ammonium sulfate —Washing —Depyrogenation • Storage conditions: — High humidity |
• Drug substance • Formulation: — Acetate, citrate, phosphate buffers — Sodium salts of organic acids, e.g., gluconate, malate, succinate, tartrate — High ionic strength, e.g., >0.1 M of alkaline salts — Complexing agents, e.g., EDTA — High pH, e.g., >8.0 • Terminal sterilization • Labeled storage conditions (refrigerated or controlled room temperature) • Shelf life |
EVALUATION OF THE INNER SURFACE DURABILITY
Each lot of Type I, II, or III glass containers received by a pharmaceutical manufacturer must comply with the Surface Glass Test in chapter  660
660 . This test provides an indication of inner surface chemical durability but does not appear to provide a clear direct correlation with the propensity to form glass particles or to delaminate. The alkalinity value represents the sum of all the internal surfaces of the container, and although this is representative for molded containers, tubular glass vials can have different degrees of surface chemical durability, depending on the location (e.g., just above the heel versus the side wall). A low surface alkalinity value can be obtained from containers treated with ammonium sulfate but the treatment itself may reduce the inner surface chemical durability, dependent upon the drug product formulation used to fill the vial. The most important variable that affects the surface durability is the drug product itself, and because it uses water as the extracting medium, the Surface Glass Test does not take this into consideration. Therefore, the Surface Glass Test represents only a first step in quality control of surface chemical durability, and additional screening methods should be used to demonstrate the suitability of vials for a formulation from a particular source before formal stability studies begin.
. This test provides an indication of inner surface chemical durability but does not appear to provide a clear direct correlation with the propensity to form glass particles or to delaminate. The alkalinity value represents the sum of all the internal surfaces of the container, and although this is representative for molded containers, tubular glass vials can have different degrees of surface chemical durability, depending on the location (e.g., just above the heel versus the side wall). A low surface alkalinity value can be obtained from containers treated with ammonium sulfate but the treatment itself may reduce the inner surface chemical durability, dependent upon the drug product formulation used to fill the vial. The most important variable that affects the surface durability is the drug product itself, and because it uses water as the extracting medium, the Surface Glass Test does not take this into consideration. Therefore, the Surface Glass Test represents only a first step in quality control of surface chemical durability, and additional screening methods should be used to demonstrate the suitability of vials for a formulation from a particular source before formal stability studies begin.
Predictive Screening Methods
Screening methods help evaluate glass containers from different vendors (molded or tubular), glass formulations (e.g., 32–33 or 48–56 expansion for tubular glass), and post-formation treatments. Screening also establishes lot-to-lot variation from individual vendors during the drug development process, as well as lot-to-lot variations for products that have been shown to have a particular propensity to form glass particles or to delaminate. Screening methods can use a number of different technologies to examine three key parameters: visual examination and chemical profile of the inner surface layer, the amount and identity of extracted elements in solution, and the number of subvisible and visible particles in solution. Taken together, these elements are assessed by predictive tests for formation of glass particles and delamination, processes that reflect reduced durability. Predictive tests should look for precursors that lead to delamination rather than looking only for glass lamellae, and should be able to quickly provide predictive indication of surface durability. This makes the tests useful not just for vendor selection but also for evaluation of individual lots if necessary. Some of the more commonly used analytical techniques for evaluating the three key parameters are shown in Table 3.
Table 3. Analytical Techniques for Screening Studies
| Parameter | Test Parameter | Instrumentation |
|---|---|---|
| Glass inner surface | • Degree of surface pitting • Chemical composition as a function of depth |
• DIC Microscopya or EMb
• SIMSc |
| Extracted elements in solution | • Conductivity/pH • SiO2 concentration |
• Conductivity/pH meter • IC–MSd or ICP–OESe |
| Lamellae and visible and subvisible glass particles | • Presence of lamellae and visible particles • Lamellae or particle number and size • Lamellae or particle morphology and composition |
• Visual inspection • Particle size analyzer • SEM–EDXf |
|
a
Differential interference contrast microscopy.
b
Electron microscopy.
c
Secondary ion mass spectrometry.
d
Inductively coupled plasma–mass spectrometry.
e
Inductively coupled plasma–optical emission spectrometry.
f
Scanning electron microscopy–energy-dispersive X-ray spectroscopy.
|
||
Aggressive Screening Conditions
In selecting an appropriate primary glass container for pharmaceutical liquids, analysts should consider two approaches. The first is a series of accelerated temperature exposures using aggressive conditions that establish, in rank order, the chemical durability of the container without any specific reference to a given compound. Such testing can be helpful when selecting a packaging system for which the most chemically durable glass is desired. This testing also can be helpful in determining if changes in glass quality have occurred or in assessing processing changes that have been made by the primary container manufacturer. Table 4 provides three examples of model systems that could be used for this assessment. Other model systems may be developed by the end users.
Table 4. Formulations and Conditions Used to Accelerate Delamination
| Formulation | 0.9% KCl pH 8.0 |
3% Sodium Citrate pH 8.0 |
20 mM Glycine pH 10.0 |
|---|---|---|---|
| Conditions | 1 h at 121 1 or 2 cycles |
24 h at 80 |
24 h at 50 |
Screening Strategy for Drug Products
Indicators include the appearance of a pitted, fractured inner surface particularly around the heel of the vial instead of a smooth surface, as well as a number of changes in the test solution, especially increases in SiO2 concentration, the number of subvisible particulates in the solution, and a change in pH.
If the purpose of the glass screening is to determine the suitability of a given glass container for a specific product, the testing proposed in Table 4 is insufficient. The exposure conditions are too harsh and do not provide a direct link to the product itself. In these instances, accelerated conditions are still relevant, but they must link to the relevant conditions for the given product. For example, if a product will be stored at 5 and the appropriate accelerated conditions are 30
and the appropriate accelerated conditions are 30 , then testing should occur at 30
, then testing should occur at 30 . Many products or formulations cannot withstand the elevated temperatures or high pH shown in Table 4. In addition, false positive testing results could be obtained because the unusually high temperatures shown in Table 4 could cause signs of delamination, but moderate exposure at 30
. Many products or formulations cannot withstand the elevated temperatures or high pH shown in Table 4. In addition, false positive testing results could be obtained because the unusually high temperatures shown in Table 4 could cause signs of delamination, but moderate exposure at 30 would produce no evidence of glass incompatibility.
would produce no evidence of glass incompatibility.
Because lower temperatures are required for actual product testing, the duration of testing must be longer, ranging from weeks to months. A larger number of vials also is appropriate for this scenario because the goal of the testing is to ensure the results are representative of the quality of the glass that will be used for the drug product. Table 5 shows some of the conditions that could be used for testing with a specific product.
Table 5. Screening Strategy for Glass Vials
| Stress Test | Water Control | Drug Product Control |
|---|---|---|
| • Vials: washed, depyrogenated • Filled with Stress Test solution • Accelerated time and temperature treatment conditions |
• Vials: washed, depyrogenated • Filled with Water for Injection • Autoclave if applicable to Drug Product • Accelerated Drug Product stability storage conditions |
• Vials: washed, depyrogenated • Filled with Drug Product • Autoclave if applicable • Accelerated Drug Product stability storage conditions |
CONCLUSIONS
Evaluation of the internal surface of glass containers begins with the Surface Glass Test, which uses water as the extracting medium. A low value is not always an indicator of a durable inner surface if the results are obtained using surface treatments (e.g., ammonium sulfate). Such treatments can lead to a silica-rich inner surface layer that represents a weakened glass structure, and risk of delamination increases when the vial is filled with formulations that contain aggressive agents such as organic acids, EDTA, or solutions that have high ionic strength or high pH. The screening methods and strategies described in this chapter can assist in the evaluation of glass containers from different suppliers or on a lot-to-lot basis and can provide an indication of the propensity of the selected formulation to cause delamination over time. Selection of glass vials intended to contain a drug product with one or more of the formulation risk factors identified in Table 2 should undergo particular scrutiny.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Desmond G. Hunt, Ph.D.
Senior Scientific Liaison (301) 816-8341 |
(GCPS2010) General Chapters - Packaging Storage and Distribution |
USP38–NF33 Page 1620
Pharmacopeial Forum: Volume No. 38(4)