SCOPE
The scope of this chapter is limited to the virus testing performed on human plasma for the further manufacture of pharmaceuticals, which are referred to as plasma-derived products (see Virology Test Methods  1237
1237 for virus testing of other therapeutic products). These types of plasma include either source plasma collected by apheresis or recovered plasma obtained from whole blood collection or as a byproduct in the production of blood components. In all cases, the source material is obtained through voluntary donations. The following topics are specifically excluded from the scope of this chapter:
for virus testing of other therapeutic products). These types of plasma include either source plasma collected by apheresis or recovered plasma obtained from whole blood collection or as a byproduct in the production of blood components. In all cases, the source material is obtained through voluntary donations. The following topics are specifically excluded from the scope of this chapter:
-
Virus testing of nonhuman blood or plasma; for example, fetal bovine serum (see Bovine Serum
 1024
1024 for more information on testing this material), which may be used in the production of biological or recombinant therapeutics
for more information on testing this material), which may be used in the production of biological or recombinant therapeutics
- Virus testing of human-derived whole blood, blood components used for transfusion, and materials in tissue and organ banks
-
Testing for nonviral organisms; for example, bacteria, fungi, and parasites (some of these topics are discussed in Sterility Tests
 71
71 ), or the causative agent of transmissible spongiform encephalopathy.
), or the causative agent of transmissible spongiform encephalopathy.
This chapter introduces the virus testing that is performed on plasma used for the production of therapeutic proteins. Topics that are addressed include:
- The rationale for implementing tests for viruses
- The types of testing applied to plasma donations destined for further manufacture
- The current regulatory environment for such virus testing
The chapter also includes an Appendix that contains pertinent regulatory guidances and supporting references.
INTRODUCTION
Human-plasma-derived products are used to treat coagulation disorders, primary immune deficiency, and congenital emphysema as well as other diseases (see Human Plasma  1180
1180 ). Because these products are manufactured from pooled human plasma donations, the presence of blood-borne viral pathogens from individual plasma donations can potentially contaminate the resulting final products manufactured from a large pool of donations and thus transmit the virus to many recipients. Sufficient measures must be taken to ensure that these products are as safe as possible. In order to minimize the risk of transmission of viruses by these products, manufacturers use several strategies, which include:
). Because these products are manufactured from pooled human plasma donations, the presence of blood-borne viral pathogens from individual plasma donations can potentially contaminate the resulting final products manufactured from a large pool of donations and thus transmit the virus to many recipients. Sufficient measures must be taken to ensure that these products are as safe as possible. In order to minimize the risk of transmission of viruses by these products, manufacturers use several strategies, which include:
-
Selection and management of the donors (see also
 1180
1180 )
)
-
Selection and management of donations or units (see also
 1180
1180 )
)
- Testing for infectious viral pathogens in plasma in the form of samples of individual or pooled donations and fractionation pools (defined for the purposes of this document as the first homogenous pool or early production intermediate suitable for testing and representative of the material to be used for product manufacturing)
-
Donor-screening methods, which also include a look-back procedure for the quarantine and destruction of unused, previously donated units from an infected donor (see also
 1180
1180 )
)
-
Incorporation of validated virus inactivation and removal steps (pathogen-reduction steps) into the manufacturing processes (see also
 1180
1180 )
)
-
Monitoring and investigating adverse events in recipients of final products, both hemovigilance and pharmacovigilance (see also
 1180
1180 )
)
- Adherence to Good Manufacturing Practices at all levels of the production process as a strategy to reduce risk of virus transmission (see GAO-HEHS-98-205, 21 CFR Part 606, and WHO Technical Report Series 941 cited in the Appendix).
Plasma used for further manufacture can be either source plasma or recovered plasma. In the United States, licensed human plasma products are derived mainly from source plasma. Because plasma for further manufacture is obtained by pooling a large number of donations, there is a risk of viral contamination of the pool, thus resulting in a much higher potential risk of virus transmission to multiple recipients than is the case for blood for transfusion. The manufacturing process, which is used to purify and concentrate the desired protein, is not capable of completely removing the viral load, and therefore validated virus-reduction steps capable of effectively reducing transfusion-transmissible viruses in the starting material are included in the manufacturing process. A detailed discussion of virus inactivation and removal procedures for pathogen reduction can be found in the 2004 WHO Technical Report Series 924 cited in the Appendix.
Approaches for screening plasma for further manufacture can be categorized into two groups: donor-screening and in-process testing methods. The donor-screening method takes into account not only the plasma-derived end product but also the plasma donor. This category of testing typically is required for blood-transmissible viruses such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV). Virus transmission is a major public safety concern, because infections with these highly pathogenic viruses typically progress to chronicity. During donor screening the objectives of the test laboratory or manufacturer are not only to identify positive units for destruction before production pooling but also to identify and notify infected donors. Donors who test positive for HCV or HIV are permanently deferred from donating both blood and plasma. Although fewer than 5% of HBV-infected adults develop persistent asymptomatic infection (i.e., a carrier state), HBV-positive donors are deferred permanently. Collection of source plasma from donors who are convalescing from HBV is sometimes permitted for further manufacturing into plasma-derived products such as Hepatitis B Immune Globulin (Human) [21 CFR 610.41(3)].
In contrast to viruses that are associated with donor screening, viruses such as hepatitis A virus (HAV) and parvovirus B19 (B19V) usually cause self-limiting infections in immunocompetent individuals, and thus manufacturers use in-process nucleic acid amplification technology (NAT) testing that results in only the removal of plasma units with high levels of virus before pooling for production. In these cases, there is no donor-management procedure and hence no requirement to inform the donor of the result. This approach focuses primarily on the product, not the donor, because some recipients of these products are susceptible to an infection that may occur if the pathogens were present in the plasma-derived product. Donor and donation-management procedures are well developed in the blood and plasma industry, and more details on these specific topics can be found in  1180
1180 . Other permanent or temporary donor-deferral criteria are in place to avoid donations from potentially infected donors based on the epidemiological surveillance of a country or region or donor population for transfusion-transmissible infections relevant to the safety of blood components.
. Other permanent or temporary donor-deferral criteria are in place to avoid donations from potentially infected donors based on the epidemiological surveillance of a country or region or donor population for transfusion-transmissible infections relevant to the safety of blood components.
Viruses that greatly affect public health, such as HIV, HBV, and HCV, are detected by serological assays that measure either a viral antigen, such as hepatitis B surface antigen (HBsAg), or an antibody, such as anti-HCV or anti-HIV-1/2 antibodies, in infected donors and associated donations. These immunoassays for detecting viral markers in plasma donations must be sensitive and able to detect a viral infection as early as possible following infection in order to identify and exclude potentially infectious donations.
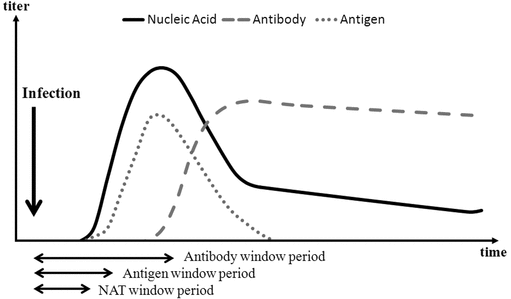
There is a finite time period, known as the window period, between the infection of a donor and the time at which the test method can detect the antibody response to the virus, the viral antigens, or the viral nucleic acid. This window period varies from disease to disease as well as from person to person. The window period can be effectively “shortened” by changing from a test based on detecting antibodies to one based on detecting the virus directly, namely the viral antigen or, especially, the viral nucleic acid (see Figure 1), thereby interdicting donations that contain transfusion-transmissible viruses. Tests for detecting the viral nucleic acids (i.e., NAT tests) were introduced in the 1990s. NAT tests are sensitive and can considerably shorten the window period (see Figure 1). In a later development after B19V transmission incidents involving plasma-derived products, NAT tests were initiated to interdict high-titer donations, thereby decreasing the B19V virus load in manufacturing pools.
Both source plasma and recovered plasma are tested by serological and NAT tests that are approved by competent regulatory authorities or, in the case of in-process testing, are validated to manufacturers' requirements. Plasma units found acceptable by these tests are combined into large pools, called fractionation pools, for manufacturing of plasma-derived products. The fractionation pool size can vary from several hundred donations (typically used for the production of specific immunoglobulins) to several thousand donations (used, for example, for the manufacture of albumin). Finally, the fractionation pools are retested for the target viruses. Testing of the plasma donations, at the individual or minipool level, and the fractionation pools are two of the elements that manufacturers put into place to maintain the safety margins of these products. Serology testing is performed on the individual donations. In contrast to serology tests, current NAT tests are highly sensitive and specific; therefore, manufacturers, in addition to testing individual donations, test minipools made up of equal volumes of each donation. Currently, the minipool size used for NAT testing varies from 6 to 512 donations. The high sensitivity of NAT tests also allows earlier virus detection compared to an antigen- or antibody-based test, thereby reducing the average length of the window period.
Plasma-derived products are produced from tested fractionation pools and are further manufactured by using a combination of fractionation and purification steps. These steps may have some inherent potential to remove or inactivate viruses and thus reduce viral contaminants that may have been present in the starting plasma. Nevertheless, manufacturing of plasma-derived products also includes dedicated steps designed solely to inactivate (e.g., by solvent-detergent treatment) or remove (e.g., by virus filtration) potential viral contaminants.
RATIONALE FOR VIRUS TESTING OF PLASMA FOR FURTHER MANUFACTURE
Historically, virological test methods have been used for detecting viral antigen or antibodies in clinical settings for disease diagnosis, intervention, and containment. Subsequently, these methods were adapted to screen blood and plasma donations with high sensitivity and specificity for transfusion-transmissible viruses. In order to develop a new virus screening test, scientists must know the biochemical properties of a new emerging pathogen (e.g., the nucleic acid sequence) for the development of an NAT test or the protein (e.g., antigen) for immunological tests. When implementing such a test for a given pathogen, the public health implications of positive test results and the potential for early intervention and treatment of the disease have to be considered. In addition, the availability of plasma for further manufacture and the effects of the virus on the safety of the finished product should be taken into consideration.
The emergence of a viral pathogen in the donor population could result in a considerable virus load in the plasma donations and in the resulting fractionation pools. For viruses such as HBV, HCV, HIV-1, and HIV-2 that can cause chronic diseases with potential public health effects, all donations positive for one or more of these viruses, irrespective of the virus titer, must be removed, and the donor must be informed. Some viruses such as B19V are prevalent in the population (as many as 1 in 5000 individuals may be infected during an epidemic period) and can be present at high virus titers in infected individuals. Thus, the removal of all B19V-positive donations could lead to a shortage of plasma. Instead, in-process NAT testing is done to interdict high-titer donations and thereby limit the B19V load in the manufacturing pool. The rationale for such screening is that B19V causes a self-limiting infection in most immunocompetent individuals. Following recovery, such individuals have neutralizing antibodies to B19V. Seroconversion occurs early in life, because B19V infection is common in childhood, and approximately 50% of 15-year-old adolescents have B19V antibodies. Infection of susceptible individuals continues throughout adult life, and B19V seroprevalence increases with age. The B19V neutralizing antibodies present in a plasma pool and the validated virus reduction steps included in the manufacturing process ensure that the inclusion of such donations does not compromise either the safety of the plasma-derived products or the availability of plasma for further manufacture.
In some instances it may not be necessary or feasible to test for a blood-borne virus. For example, testing of plasma for cell-associated viruses such as Human T Lymphotropic Virus (HTLV) types I and II, which present with no or with only limited virus load in plasma (but with a considerable virus load in whole blood donations) is not required. Similarly, testing for West Nile Virus (WNV), a member of the Flaviviridae family, is unnecessary because the virus load is low during the asymptomatic window period, the prevalence in the donor population is low (resulting in a low virus load in a plasma pool for fractionation), and WNV can be effectively inactivated by the manufacturing process as demonstrated by validation studies using relevant Flaviviridae model viruses. Therefore, WNV NAT testing for plasma (source and recovered) for further manufacture is not required. However, in the United States, the Food and Drug Administration (FDA) recommends WNV NAT testing for blood and blood components for transfusion because of the epidemiological situation and the risk of WNV transmission by blood components (see the FDA Guidance for Industry cited in the Appendix).
Other pathogenic viruses such as influenza viruses and severe acute respiratory syndrome coronavirus (SARS-CoV) are associated with clinical disease after a short incubation period and have a low or no virus load during the asymptomatic window period. No transmission by blood transfusion or plasma-derived products has been reported for these viruses. Furthermore, the manufacturing process for plasma-derived products has been shown to effectively inactivate influenza viruses. Therefore, NAT testing of plasma is not required for these viruses.
For a virus with a high prevalence in the donor population but without known clinical implications, no screening program, neither NAT nor serology, is required because the majority of donors would no longer be eligible to donate, thereby threatening the supply of blood and plasma and of plasma-derived products. Viruses that fall in this category are Torque Teno Virus (TTV), which is present in greater than 80% of the general population, and GB virus C (GBV-C, previously known as hepatitis G virus, HGV).
Newly emerging pathogens such as hepatitis E virus (HEV) can potentially enter the blood and plasma donor population, resulting in viral infections in recipients of blood and plasma-derived products. Monitoring the emergence of such agents is a continuous effort that involves academia, public organizations that monitor health and develop early warning systems, regulatory agencies, and industry. Currently, several epidemiological surveillance systems are in place and include hemovigilance or biovigilance to address the potential risk of emerging pathogens to the recipients of blood and plasma-derived products. This risk can be mitigated by appropriate measures, such as donor deferral because of geographic risk and risk behaviors, the testing of donations if appropriate, and the inclusion of virus-reduction steps for a wide range of enveloped and nonenveloped viruses during the manufacturing process.
APPROACHES TO TESTING
Virological screening assays are designed to detect antibodies, antigens, or nucleic acid sequences of the infectious virus via serological and NAT testing. Sensitive virological test methods are a prerequisite for the quality control of fractionation pools in order to interdict and discard infected donations before manufacturers process these donations into pools to produce plasma-derived products.
All assays used to screen blood or plasma donations should be designed for their intended use and should meet the performance requirements specified by the Clinical and Laboratory Standards Institute (CLSI) guidelines for qualitative and quantitative tests. Assays also should comply with guidance from regulators, such as the European Common technical specifications for in vitro diagnostic assays (see Appendix). Associated calibrators or control materials must be traceable to reference material of a higher order or to reference measurement procedures. The Appendix includes FDA guidance documents pertaining to the manufacture and clinical evaluation of these assays and the use of controls. U.S. and EU requirements or recommendations for tests for screening plasma for further manufacture are described in the Regulatory Environment section.
Serological (Immunological) Assays
Serological assays detect antibodies, antigen, or a combination of both. Antibody-detection assays usually are performed by incubating an immobilized virus antigen (virus lysate or, more common currently, virus proteins produced by recombinant protein technology) with a plasma sample. If antibodies specific to the viral protein are present in the sample, they bind to the target antigen. The virus-specific, bound antibody is in turn incubated with a labeled secondary antibody that is specific for the virus-specific, bound antibody. The label yields a signal that then is detected. Alternatively, for the measurement of a viral antigen present in a sample, immobilized antibodies specific for the viral antigen first are incubated with a plasma sample, a labeled antibody (often a monoclonal antibody) against the virus protein is added, and the mixture is incubated. For more details about these assays, see Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)  1103
1103 .
.
Nucleic Acid Amplification Technology Tests
NAT is a collective term for the various methods that are used to amplify and detect the specific genomic sequences in various sample types. These methods include polymerase chain reaction (PCR), transcription-mediated amplification (TMA), and branched DNA (bDNA) and are detailed in Nucleic Acid-Based Techniques—General  1125
1125 , Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing
, Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing  1126
1126 , and Nucleic Acid-Based Techniques—Amplification
, and Nucleic Acid-Based Techniques—Amplification  1127
1127 . NAT tests typically use gene-specific oligonucleotides (e.g., primers, probes), enzymatic amplification reagents (e.g., buffers, nucleotides, enzymes, cofactors), and a method that allows the detection of the resulting amplification products. Currently, NAT tests for HBV, HCV, HIV, B19V, and, in some cases, HAV, are used to screen plasma for further manufacture.
. NAT tests typically use gene-specific oligonucleotides (e.g., primers, probes), enzymatic amplification reagents (e.g., buffers, nucleotides, enzymes, cofactors), and a method that allows the detection of the resulting amplification products. Currently, NAT tests for HBV, HCV, HIV, B19V, and, in some cases, HAV, are used to screen plasma for further manufacture.
For high-throughput testing, which is desired for the testing of plasma donations used for further manufacture, NAT offers distinct advantages over serological testing. First, because of the high sensitivity of these methods, samples of plasma donations can be combined into pools (minipools) that allow simultaneous testing of multiple samples, in contrast to serological assays that are performed on individual samples. Although the operational logistics used in donation minipool testing are more complicated than those involved in testing individual donations, the minipool approach generally allows an improved turnaround time for the release of negative samples compared to the traditional nonpooling method. A reactive minipool is deconstructed to identify any positive donation(s). However, because of the dilution of virus in any given donation, minipool testing has an inherently decreased sensitivity compared to individual donation testing.
Table 1 summarizes the potential viral load, which could be avoided by NAT testing, in a fractionation pool caused by the inclusion of a single serological-window-period donation for the five major transfusion-transmitted viruses.
Table 1
| Virus | Potential Viral Load in Fractionation Pool Caused by Contamination with One Window-Period Donation (Approximate Values)a |
|---|---|
| HBV | 8 ×105 IU |
| HCV | 8 ×1010 IU |
| HIV-1 | 8 ×109 IU |
| HAV | 8 ×109 IU |
| B19V | 8 ×1014 IU |
|
a
Assuming one plasma donation is approximately 800 mL. References supporting these values are found in the Appendix.
|
|
REGULATORY ENVIRONMENT
Regulatory agencies have the goal of ensuring that plasma-derived products are safe with respect to risk from blood-borne pathogens. In addition to regulating the final products, regulators also oversee the assays used to test for infectious agents and set policies about how those tests will be used. Although regulatory agencies in the United States and Europe share the common goal of safety, they use different legal structures and strategies. In general, the hierarchy of regulatory documents is similar. Both start with laws that set the definitive requirements for donor selection and plasma screening.
In the United States, the primary laws that regulate plasma-derived products and the assays that test their safety are the Public Health Service (PHS) Act and the Federal Food, Drug, and Cosmetic (FD&C) Act. The PHS Act addresses biologics and communicable disease controls, and the FD&C Act addresses drugs and medical devices. Donor-screening tests are licensed under the PHS Act rather than being cleared or approved under the medical device provisions of the FD&C Act. Testing requirements for communicable disease agents, including viral pathogens such as HBV, HCV, and HIV, are required under 21 CFR, including not only test requirements (21 CFR 610.40) but also donor deferral (21 CFR 610.41) and look-back requirements (21 CFR 610.46 through 610.48). If a plasma or blood donation is reactive in one of the screening tests, especially in the donor-screening tests for HBV, HCV, or HIV, supplementary or confirmation tests should be conducted to clarify whether the donor is infected [21 CFR 610(b)]. The donor must be informed (21 CFR 630.6) and should be excluded from donating blood or plasma (temporarily or permanently according to 21 CFR 630.6), and manufacturers should initiate a look-back procedure (21 CFR 610.40–48). For HIV and HCV, the procedure includes not only the quarantine and destruction of unused, previously donated units from an infected donor, but also the further testing of the donor and notification of the recipients of the blood and blood components (21 CFR 610.47–48). The look-back period can be as long as 1 year (21 CFR 610.46–48).
The responsibility for legislation of the European Union (EU) is shared between the EU and the European Member States. The European Commission (EC) is responsible for the regulation of the common European market and thus is responsible for medicinal products for human use, whereas the Member States are responsible for health care, which includes the supply of hospitals with blood components such as plasma and cellular components for transfusion. Because the EC is responsible for the regulation of medicinal products derived from human blood or plasma, it is, as a consequence, also responsible for the regulation of plasma for further manufacture. The laws of the EU are found in regulations and directives from the EC and in the binding monographs of the European Pharmacopoeia (Ph. Eur.).
Additional tests and specifications for plasma for further manufacture have been developed as part of the Plasma Product Therapeutics Association (PPTA) Voluntary Standards Program. The PPTA Quality Standard for Excellence, Assurance, and Leadership (QSEAL) includes additional routine testing of blood and plasma donations or plasma pools for HCV RNA, HIV RNA, HBV DNA, HAV RNA, and B19V DNA. Companies certified by PPTA under the QSEAL program have implemented this testing.
Testing of Plasma for Further Manufacture
In the United States, all virus tests intended for donor screening, such as HBV, HCV, and HIV, are regulated as biologics and are subject to clinical validation and licensure by FDA's Center for Biologics Evaluation and Research (CBER). Clinical specificity must be evaluated and demonstrated with healthy donors and follow-up testing when applicable, and clinical sensitivity should be evaluated and demonstrated with high-risk donors and follow-up testing. Use of reference panels (from FDA or a designated source) is needed for release of each lot of kits intended for market distribution. In-process tests such as NAT testing for HAV or B19V do not require clinical trials to demonstrate assay effectiveness. However, the manufacturers of plasma-derived products should perform preclinical validation and should submit data for review and approval by CBER as analytical procedures for plasma-derived products.
In the European Union, donation screening tests are regulated as medical devices. Specifically, Directive 98/79/EC outlines requirements for the approval of tests or test kits, which require the CE mark before marketing and use for testing. The CE mark confirms that the test or the test kit meets specified quality criteria. Screening tests for manufacturing pools must be validated by the end user following specific guidelines. As in the United States, in-process tests are not licensed, and the end user is responsible for validating the test.
In Europe, in addition to virus screening of plasma pools by the manufacturers of plasma-derived products, screening for defined viruses is part of the official batch-release procedure. The EC and the Council of Europe agreed in May 1994 to create a network of Official Medicines Control Laboratories (OMCLs). The OMCLs perform tests on each batch of plasma-derived medicinal product, including virus testing of the fractionation pool used to produce the batch. All required tests are performed and documented in the European Batch Release Certificate that is accepted by each Member State as the basis for placing the product on the market. In order to comply with the sensitivity limits set for NAT testing, minipools of various sizes (6–512 donations) are tested by the manufacturer or by the blood donation centers where the collection and testing of blood or plasma is performed. Only donations that meet the requirements are used for pooling.
Serological Tests
fda requirements or recommendations
An individual donation of source plasma or recovered plasma derived from whole blood must be tested for HBsAg, anti-HIV-1, anti-HIV-2, and anti-HCV, but not for anti-HBc, anti-HTLV-I, and anti-HTLV-II by FDA-licensed serological tests intended for donor screening (Table 2). A reactive donation must be further tested by a supplemental (i.e., additional, more specific) test that has been approved for such use. Even with the implementation of corresponding NAT tests, serological testing of each donation still must be performed.
Currently, FDA recommends using licensed donor-screening kits that are capable of detecting anti-HBsAg, the antibody capable of neutralizing HBV, at 0.5 ng/mL or less. Whole blood sometimes is tested for anti-HBc, but because anti-HBsAg often occurs with anti-HBc, plasma for further manufacture is not required to be tested for anti-HBc. Therefore, although recovered plasma is derived from whole-blood donations that might have been tested for anti-HBc, it can be shipped for further manufacturing regardless of the test results.
FDA first recommended standardized anti-HIV-1 donor-screen tests in 1989 in a draft Points to Consider document that described test kit manufacture and the preclinical and clinical studies needed for licensure, and this recommendation generally can be applied to other serological tests. Since the availability in 1991–1992 of licensed serological kits for simultaneous detection of antibodies to HIV-1 and HIV-2, FDA further recommends the use of either a licensed combined test or two separate licensed tests for donor screening.
FDA licensed an anti-HCV test containing multiple recombinant antigens in 1992, and a subsequent guidance recommended that all donations for blood and blood components intended for transfusion and source plasma intended for further manufacture be screened by an FDA-licensed test for anti-HCV.
eu requirements
Requirements for collection of blood and plasma, for selection of donors, and for testing of donations in Europe were released in 2003. Directives 2002/98/EC and 2003/63/EC contain donor-selection criteria and testing requirements for blood and plasma independent of its use. The requirements of the directives are standards for plasma for further manufacture but can be extended by a Member State for the regulation of blood components. An overview is provided in the Reports of the European Committee (Partial Agreement) on Blood Transfusion (CD-P-TS). The report indicates that in addition to the serological standard tests that are obligatory for the testing of plasma for fractionation (summarized in Table 2), testing for HIV antigen, anti-HBc antibodies, HCV antigen, anti-HTLV-I, and anti-HTLV-II antibodies is required in some Member States, and testing for antibodies against cytomegalovirus is performed in certain cases. However, these additional rules are applicable only if blood components for transfusion (erythrocytes, platelets, or plasma) are produced.
The test regime required for donations used for production of plasma derivatives are summarized in the Ph. Eur. monograph Human Plasma for Fractionation (0853). Only licensed tests or test kits can be used for donor screening. Licensed tests have a CE mark, which confirms that the quality of the test meets predefined criteria (e.g., an HBV screening test must detect HBsAg in a concentration of 0.5 ng/mL or less). The most important quality attributes, namely specificity and sensitivity, are tested in clinical trials using donor samples (minimum of 5000 samples) and clinical samples (minimum of 200 samples). Sensitivity of the tests must be demonstrated with positive samples (minimum of 400 samples) and with seroconversion panels (minimum of 20 panels).
HBsAg tests and antibody tests against HIV Types 1 and 2 also are used for the testing of fractionation pools. The plasma manufacturer should demonstrate that the test is qualified for this use and meets the requirements laid down in the appropriate guidelines of the European Medicines Agency.
The current test regime is discussed regularly among EU Member States and may be subject to change if necessary because of the epidemiological situation. If changes in the requirements are made, the Ph. Eur. monograph Human Plasma for Fractionation (0853) and the product-specific monographs will be adopted accordingly (e.g., a change in the monograph Human Plasma (Pooled and Treated for Virus Inactivation) (1646) is proposed; it requires testing for HEV by NAT).
NAT Tests
As described previously, only licensed serological tests that use antibody- or antigen-detection technology are required to screen plasma in single-donation format. However, NAT testing generally can detect evidence of viral infection at an early stage, and FDA licensed NAT tests for HIV-1 and HCV in 2001 for source plasma donors and in 2002 for whole-blood collections. Thus NAT tests are used to screen plasma donations, generally in a minipool format, using pool sizes that depend on the analytical sensitivity of the NAT test and, in some cases (HCV and B19V), the fractionation pool. In general, plasma donations are screened in a minipool format in which the pool size depends on the analytical sensitivity of the NAT test. The size of the minipool used for source plasma donations generally is much larger than that for blood donation testing (as large as 512 compared with 96 for blood donations). The turnaround time required for retesting a reactive pool in order to identify the reactive donation is less critical for plasma compared with that for blood for transfusion because some blood components such as platelets have a short shelf life.
fda requirements or recommendations
To adequately and appropriately reduce the risk of transmissions of HIV-1, HCV, and HBV, FDA-licensed NAT tests are required for donor screening. A list of FDA-licensed, donor-screening NAT tests and serological tests is updated as needed and is available on the FDA website.
FDA's initial guidance for HIV NAT in 1999 recommended standards for the manufacture and clinical evaluation of tests to detect nucleic acid sequences of HIV-1 and HIV-2 for licensure. This guidance provided some of the major regulatory and scientific guidance for NAT assays not only for HIV but also for other transfusion-transmitted viruses. Since then FDA has revised the requirements for the analytical sensitivity of HIV-1 and HCV NAT tests as 100 IU/mL for HIV-1 RNA and HCV RNA when tested in a minipool or as 10,000 IU/mL HIV-1 RNA or 5000 IU/mL HCV RNA when tested in an individual donation. FDA's 2004 guidance on NAT screening of HIV-1 and HCV in donor whole blood, blood components, and source plasma and a further guidance in 2010 contain recommendations about testing, product disposition, and donor deferral and reentry. The latter supersedes earlier recommendations for reentry of donor deferral and reentry because of serological testing results for anti-HIV-1 and anti-HCV.
The source plasma industry has voluntarily implemented HBV NAT testing in minipool format. Several FDA-licensed HBV NAT tests for donor screening are available. In 2012 FDA finalized a guidance recommending the use of HBV NAT on pooled and individual samples from donors of whole blood and blood components for transfusion or for further manufacture, including recovered plasma and source plasma. The guidance recommends an NAT test sensitivity of 100 IU/mL for testing individual donations of whole blood and blood components intended for transfusion. Because of the virus-reduction step(s) used during the manufacturing of plasma-derived products and the presence of neutralizing anti-HBsAg in the manufacturing pools, FDA recommends a NAT test sensitivity of 500 IU/mL for individual donations when manufacturers test minipools of plasma for further manufacture. The guidance also contains recommendations about product testing and disposition, donor management, methods of donor requalification, and product labeling. The guidance also supersedes the relevant recommendations based on HBsAg and anti-HBc serological testing results.
In 2009 FDA issued a final guidance for B19V NAT testing following a postmarket surveillance study report of a B19V transmission incident associated with solvent and detergent-treated (S/D-treated) pooled plasma. The guidance recommends the use of B19V NAT as an in-process test for plasma for further manufacturing to ensure that the level of B19V DNA in fractionation pools does not exceed 104 IU/mL. The guidance document recommends that the primers and probes selected for a B19V NAT test should detect all known genotypes of the virus. The WHO International B19 Genotype Panel containing three genotypes is available for validation purposes. Currently, in-process HAV NAT testing is widely implemented by fractionators who use source plasma as starting plasma, but FDA has not issued a relevant guidance document. Because the in-process B19V NAT test is used to limit the virus load in the plasma pool, these tests must be capable of B19V DNA quantitation (unlike the NAT tests for HBV, HCV, HIV, and HAV, which are qualitative NAT tests).
european requirements
NAT tests used for donor screening are subject to licensing and receive the CE mark if a test meets the predefined test specifications. NAT tests for plasma pool samples must be validated according to the Ph. Eur. general test Nucleic Acid Amplification Techniques (20621). Currently, plasma for manufacture (plasma pools for fractionation) must be tested for HCV RNA by a NAT test, but there are no requirements for NAT testing for HBV DNA and HIV RNA although most plasma manufacturers voluntarily test for all three viruses. The guideline requires that a test should be able to detect all HCV genotypes. However, in view of the difficulty of obtaining rare HCV genotypes, it is sufficient that at least the most prevalent genotypes (in Europe, genotypes 1 and 3) are detected at a suitable level. Plasma should be negative when screened with a test that can detect a sample containing 100 IU/mL of HCV RNA (calibrated against the WHO HCV International Standard).
Testing for B19V DNA generally is not required but must be performed for products that are seen as a higher risk for patients if this virus is present (e.g., anti-D immunoglobulin products and plasma that is pooled and inactivated by S/D treatment). In addition, the latter product also must be tested and found nonreactive for HAV RNA, and in the future it also should be nonreactive for HEV RNA. Unlike testing for HCV and HAV where the fractionation pool should be nonreactive for these viruses, for B19V testing the virus load in the fractionation pool and pooled S/D-treated plasma should not exceed 10 IU/µL B19V DNA. The requirements are detailed in the product-specific Ph. Eur. monographs. In order to avoid a reactive pool, which would have to be discarded, NAT testing also is performed on single donations or preferentially on minipools comprising 16–512 donations. Although the requirement for B19V and HAV NAT testing is applicable only to plasma used for the manufacture of pooled S/D-treated plasma and anti-D immunoglobulin products, most plasma manufacturers voluntarily test plasma destined for manufacture of all plasma-derived products to reduce the virus load in the fractionation pools.
Table 2. FDA and EU Serology Testing Requirements for Plasma for Further Manufacture
| Screening Test | FDA | EU |
|---|---|---|
| Serological Testing of Individual Plasma Donations (Recovered and Source) |
||
| HBsAg | Required | Required |
| Anti-HBc | Not required | Not required |
| Anti-HIV-1/Anti-HIV-2 | Required | Required |
| Anti-HTLV-I/II | Not required | Not required |
| Anti-HCV | Required | Required |
| Serological Testing of the Fractionation Pool | ||
| HBsAg | Not required but widely implemented by plasma fractionators | Required |
| Anti-HIV | Not required but widely implemented by plasma fractionators | Required |
Table 3. FDA and EU NAT Testing Requirements for Plasma for Further Manufacture
| Screening Test | FDA | EU |
|---|---|---|
| NAT Testing of Plasma Donations in Minipool Format | ||
| HIV-1 RNA | Required, using tests with a sensitivity of 10,000 IU/mL for the individual donation | Not required but widely implemented by plasma fractionators |
| HCV RNA | Required, using tests with a sensitivity of 5000 IU/mL for the individual donation | Not required but recommended in order to avoid unnecessary loss of a fractionation pool (see below) |
| WNV RNA | Not required | Not required |
| HBV DNA | Required, using tests with a sensitivity of 500 IU/mL for the individual donation | Not required but widely implemented by plasma fractionators |
| B19V DNA | Required with a manufacturing pool limit of |
Required for specific products (anti-D immunoglobulin and pooled S/D-treated plasma); a manufacturing pool limit of B19V DNA |
| HAV RNA | Not required but widely implemented by plasma fractionators | Required only for S/D-treated plasma; not required for other products but widely implemented by most plasma fractionators |
| HEV | Currently not required | Testing not yet required; testing requirements will be introduced for a specific product only (pooled S/D-treated plasma). |
| NAT Testing of the Fractionation Pool | ||
| HIV-1 RNA | Not required but widely implemented by plasma fractionators | Not required but widely implemented by plasma fractionators |
| HCV RNA | Not required but widely implemented by plasma fractionators | Required; the fractionation pool must be nonreactive using a test that detects 100 IU/mL of HCV RNA. |
| WNV RNA | Not required | Not required |
| HBV DNA | Not required but widely implemented by fractionators | Not required but widely implemented by fractionators |
| B19V DNA | Required; a limit of |
Required for specific products (anti-D immunoglobulin and pooled S/D-treated plasma); a limit of B19V DNA |
| HAV RNA | Not required but widely implemented by plasma fractionators | Required only for a specific product (pooled S/D-treated plasma); the fractionation pool must be nonreactive using a test that detects 100 IU/mL. |
| HEV | Not required | Testing not yet required; testing requirements will be introduced for a specific product only (pooled S/D-treated plasma). After the requirement is implemented, the plasma pool must be nonreactive using a test that can detect 2.5 log10 IU/mL of HEV RNAa. |
|
a
The new monograph will be implemented soon. See the draft Ph. Eur. monograph Human Plasma (Pooled and Treated for Virus Inactivation (1640)). http://pharmeuropa.edqm.eu/TextsForComment/NetisUtils/srvrutil_getdoc.aspx/2L3OqDZGmCLmnDZGsHIveT6q0//1646E.pdf. Accessed 20 February 2013.
|
||
CONCLUSIONS
All the measures discussed in this chapter, along with virus-reduction steps included during the manufacturing process, ensure the safety of plasma-derived products. However, testing of plasma for further manufacture is only one of the steps taken to ensure the safety of the final plasma-derived products. Both manufacturers and regulators face continuing challenges because of the emergence of new blood-borne viruses, mutants, and variants of existing viruses not detected by current serological/NAT technology. The development of new screening tests and regulatory guidance documents depends on whether the emerging virus is a risk to the safety of plasma-derived products.
APPENDIX
Regulatory Guidances
- GAO-HEHS-98-205: General Accounting Office, Blood Plasma Safety: Plasma product risks are low if good manufacturing practices are followed.
- 21 CFR 606: Current good manufacturing practice for blood and blood components.
- WHO. Technical report, series 941, 2007: Recommendations for the production, control and regulation of human plasma for fractionation.
- FDA. Guidance for industry: use of nucleic acid tests on pooled and individual samples from donors of whole blood and blood components, including source plasma, to reduce the risk of transmission of hepatitis B virus. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM327895.pdf. Accessed 21 February 2013.
- FDA. Guidance for industry: use of nucleic acid tests to reduce the risk of transmission of West Nile Virus from donors of whole blood and blood components intended for transfusion.
- 21 CFR 610.40–610.48, Part 630 or Part 640.
- WHO. Technical report, series 924, annex 4: guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. http://www.who.int/bloodproducts/publications/WHO_TRS_924_A4.pdf. Accessed 20 February 2013.
- EMEA/CHMP/BWP/706271/2010. Guideline on plasma-derived medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109627.pdf. Accessed 25 February 2013.
- FDA. Guidance for industry: nucleic acid testing (NAT) for human immunodeficiency virus type 1 (HIV-1) and hepatitis C virus (HCV): testing, product disposition, and donor deferral and reentry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm210270.pdf. Accessed 21 February 2013.
- PPTA. Quality standards for excellence, assurance, and leadership (QSEAL). http://www.pptaglobal.org/safety-quality/standards/qseal. Accessed 21 February 2013.
- Council of Europe. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official 07 December 1998. Strasbourg, France: EMEA; 1998:1–37.
- Council of Europe. Directive 98/79/EC, Annex II List A in combination with Article 9(2), (3). Strasbourg, France: EMEA; 1998.
- Council of Europe. Commission Decision of 7 May 2002 on common technical specifications for in vitro-diagnostic medical devices (2002/364/EC); Official Journal L131 of 16/05/2002, p. 17–30. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:131:0017:0030:EN:PDF. Accessed 25 February 2013.
- EDQM. General European OMCL network. http://www.edqm.eu/site/General-European-OMCL-Network-46.html. Accessed 21 February 2013.
- EDQM. Heads of Medicines Agencies (HMA) Working Group on Product Testing. Principles for mutual recognition of control results. http://www.edqm.eu/medias/fichiers/NEW_Principles_for_Mutual_Recognition_of_Control_r.pdf. Accessed 21 February 2013.
- EDQM. Official Control Authority Batch Release (OCABR) for human biologicals: vaccines, blood, and plasma derivatives. http://www.edqm.eu/site/Human_Biologicals_OCABR-611.html. Accessed 21 February 2013.
- FDA. Guidance for industry: adequate and appropriate donor screening tests for hepatitis B; hepatitis B surface antigen (HBsAg) assays used to test donors of whole blood and blood components, including source plasma and source leukocytes. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm072543.htm. Accessed 21 February 2013.
- FDA. Recommendations concerning testing for antibody to hepatitis B core antigen (Anti-HBc). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/OtherRecommendationsforManufacaturers/MemorandumtoBloodEstablishments/ucm062847.pdf. Accessed 21 February 2013.
- FDA. Draft of points to consider in the manufacture and clinical evaluation of in vitro tests to detect antibodies to the human immunodeficiency virus type 1. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm080958.pdf. Accessed 21 February 2013.
- FDA. Revised recommendations for the prevention of human immunodeficiency virus (HIV) transmission by blood and blood products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Other RecommendationsforManufacturers/MemorandumtoBloodEstablishments/ucm062834.pdf. Accessed 21 February 2013.
- FDA. Revised recommendations for testing whole blood, blood components, source plasma, and source leukocytes for antibody to hepatitis C virus encoded antigen (anti-HCV). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Other RecommendationsforManufacturers/MemorandumtoBloodEstablishments/ucm062832.pdf. Accessed 21 February 2013.
- Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC. OJEU. 2003;L33:30–40. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:033:0030:0040:EN:PDF. Accessed 25 February 2013.
- Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirement for blood and blood components. OJEU. 2004;L91:25–39. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:091:0025:0039:EN:PDF. Accessed 25 February 2013.
- EDQM, Council of Europe. The collection, testing, and use of blood and blood components in Europe. http://www.edqm.eu/medias/fichiers/The_Collection_Testing_and_Use_of_Blood_and_Blood_3.pdf. Accessed 21 February 2013.
- EMEA/CHMP/BWP/298390/2005. Guideline on validation of immunoassay for the detection of hepatitis B virus surface antigen (HBsAg) in plasma pools. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003704.pdf. Accessed 25 February 2013.
- EMEA/CHMP/BWP/298388/2005. Guideline on validation of immunoassay for the detection of antibody to human immunodeficiency virus (anti-HIV) in plasma pools. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003685.pdf. Accessed 25 February 2013.
- FDA. Complete list of donor screening assays for infectious agents and HIV diagnostic assays. http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/ucm080466.htm. Accessed 21 February 2013.
- FDA. Guidance for industry: in the manufacture and clinical evaluation of in vitro tests to detect nucleic acid sequences of human immunodeficiency viruses types 1 and 2. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm077067.htm. Accessed 21 February 2013.
- FDA. Guidance for industry: use of nucleic acid tests on pooled and individual samples from donors of whole blood and blood components (including source plasma and source leukocytes) to adequately and appropriately reduce the risk of transmission of HIV-1 and HCV. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm074934.htm. Accessed 21 February 2013.
- FDA. Recommendations for the management of donors and units that are initially reactive for hepatitis B surface antigen (HBsAg) [letter]. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/OtherRecommendationsforManufacturers/MemorandumtoBloodEstablishments/ucm063011.pdf. Accessed 21 February 2013.
- FDA. Guidance for industry: nucleic acid testing (NAT) to reduce the possible risk of human parvovirus B19 transmission by plasma-derived products. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm071592.htm. Accessed 21 February 2013.
- FDA, Blood Products Advisory Committee. Nucleic acid testing of blood donors for human parvovirus B19. http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3548t1b.pdf and http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3548t1c.pdf. Accessed 21 February 2013.
Table 1 References
- Glynn SA, Wright DJ, Kleinman SH, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002.
- Iudicone P, Miceli M, Palange M, et al. Hepatitis B virus blood screening: impact of nucleic amplification technology testing implementation on identifying hepatitis B surface antigen non-reactive window period and chronic infections. Vox Sanguinis. 2009;96:292–297.
- Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelsoni AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010;84:6096–6102.
- Tobler LH, Stramer SL, Lee SR, et al. Performance of ORTHO HCV core antigen and trak-C assays for detection of viraemia in pre-seroconversion plasma and whole blood donors. Vox Sanguinis. 2005;89:201–207.
- Weimer T, Streichert S, Watson C, Gröner A. Hepatitis A virus prevalence in plasma donations. J Med Virol. 2002;67:469–471.
- Young NS, Brown KE. Mechanisms of disease: parvovirus B19. N Engl J Med. 2004;350:586–597.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Maura C Kibbey, Ph.D.
Senior Scientific Liaison, Biologics & Biotechnology (301) 230-6309 |
(GCBA2010) General Chapters - Biological Analysis |
USP38–NF33 Page 1582
Pharmacopeial Forum: Volume No. 39(4)