This chapter provides guidance and procedures used for characterization of biotechnology-derived articles by peptide mapping. This chapter is harmonized with the corresponding chapter in JP and EP. Portions of the chapter that are not harmonized with the other two pharmacopeias are marked by the symbol  . Other characterization tests, also harmonized, are shown in Biotechnology-Derived Articles—Amino Acid Analysis
. Other characterization tests, also harmonized, are shown in Biotechnology-Derived Articles—Amino Acid Analysis  1052
1052 , Biotechnology-Derived Articles—Capillary Electrophoresis
, Biotechnology-Derived Articles—Capillary Electrophoresis  1053
1053 , Biotechnology-Derived Articles—Isoelectric Focusing
, Biotechnology-Derived Articles—Isoelectric Focusing  1054
1054 , Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis
, Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis  1056
1056 , and Biotechnology-Derived Articles—Total Protein Assay
, and Biotechnology-Derived Articles—Total Protein Assay  1057
1057 .
.
INTRODUCTION
Peptide mapping is an identity test for proteins, especially those obtained by rDNA technology. It involves the chemical or enzymatic treatment of a protein, resulting in the formation of peptide fragments, followed by separation and identification of the resultant fragments in a reproducible manner. It is a powerful test that is capable of identifying single amino acid changes resulting from events such as errors in the reading of complementary DNA (cDNA) sequences or point mutations. Peptide mapping is a comparative procedure because the information obtained, compared to a Reference Standard or Reference Material similarly treated, confirms the primary structure of the protein, is capable of detecting whether alterations in structure have occurred, and demonstrates process consistency and genetic stability. Each protein presents unique characteristics that must be well understood so that the scientific and analytical approaches permit validated development of a peptide map that provides sufficient specificity.
This chapter provides detailed assistance in the application of peptide mapping and its validation to characterize the desired protein product, to evaluate the stability of the expression construct of cells used for recombinant DNA products, to evaluate the consistency of the overall process, and to assess product stability, as well as to ensure the identity of the protein product or to detect the presence of protein variant.  The validation scheme presented differentiates between qualification of the method at an early stage in the regulatory process, the Investigational New Drug (IND) level, and full validation in support of New Drug Application (NDA), Product License Application (PLA), or Marketing Authorization Application (MAA). The validation concepts described are consistent with the general information chapter Validation of Compendial Procedures
The validation scheme presented differentiates between qualification of the method at an early stage in the regulatory process, the Investigational New Drug (IND) level, and full validation in support of New Drug Application (NDA), Product License Application (PLA), or Marketing Authorization Application (MAA). The validation concepts described are consistent with the general information chapter Validation of Compendial Procedures  1225
1225 and with the International Conference on Harmonization (ICH) document Analytical Methods Validation.
and with the International Conference on Harmonization (ICH) document Analytical Methods Validation.
THE PEPTIDE MAP
Peptide mapping is not a general method, but involves developing specific maps for each unique protein. Although the technology is evolving rapidly, there are certain methods that are generally accepted. Variations of these methods will be indicated, when appropriate, in specific monographs.
A peptide map may be viewed as a fingerprint of a protein and is the end product of several chemical processes that provide a comprehensive understanding of the protein being analyzed. Four major steps are necessary for the development of the procedure: isolation and purification of the protein, if the protein is part of a formulation; selective cleavage of the peptide bonds; chromatographic separation of the peptides; and analysis and identification of the peptides. A test sample is digested and assayed in parallel with a Reference Standard or Reference Material. Complete cleavage of peptide bonds is more likely to occur when enzymes such as endoproteases (e.g., trypsin) are used instead of chemical cleavage reagents. A map should contain enough peptides to be meaningful. On the other hand, if there are too many fragments, the map might lose its specificity because many proteins will then have the same profiles.
Isolation and Purification
Isolation and purification are necessary for analysis of bulk drugs or dosage forms containing interfering excipients and carrier proteins and, when required, will be specified in the monograph. Quantitative recovery of protein from the dosage form should be validated.
Selective Cleavage of Peptide Bonds
The selection of the approach used for the cleavage of peptide bonds will depend on the protein under test. This selection process involves determination of the type of cleavage to be employed—enzymatic or chemical—and the type of cleavage agent within the chosen category. Several cleavage agents and their specificity are shown in Table 1. This list is not all-inclusive and will be expanded as other cleavage agents are identified.
Table 1. Examples of Cleavage Agents
| Type | Agent | Specificity |
|---|---|---|
| Enzymatic | Trypsin, EC 3.4.21.4 | C-terminal side of Arg and Lys |
| Chymotrypsin, EC 3.4.21.1 | C-terminal side of hydrophobic residues (e.g., Leu, Met, Ala, aromatics) | |
| Pepsin, EC 3.4.23.1 and EC 3.4.23.2 | Nonspecific digest | |
| Lysyl endopeptidase (Lys-C endopeptidase), EC 3.4.21.50 | C-terminal side of Lys | |
| Glutamyl endopeptidase; (from S. aureus strain V8), EC 3.4.21.19 |
C-terminal side of Glu and Asp | |
| Peptidyl-Asp metalloendopeptidase (Asp-N endoproteinase), EC 3.4.24.33 | N-terminal side of Asp | |
| Clostripain, EC 3.4.22.8 | C-terminal side of Arg | |
| Chemical | Cyanogen bromide | C-terminal side of Met |
| 2-Nitro-5-thiocyanobenzoic acid | N-terminal side of Cys | |
| o-Iodosobenzoic acid | C-terminal side of Trp and Tyr | |
| Dilute acid | Asp and Pro | |
| BNPS-skatole | Trp |
Pretreatment of Sample
Depending on the size or the configuration of the protein, different approaches in the pretreatment of samples can be used. For monoclonal antibodies, the heavy and light chains will need to be separated before mapping. If trypsin is used as a cleavage agent for proteins with a molecular mass greater than 100,000 Da, lysine residues must be protected by citraconylation or maleylation; otherwise, too many peptides will be generated.
Pretreatment of the Cleavage Agent
Pretreatment of cleavage agents, especially enzymatic agents, might be necessary for purification purposes to ensure reproducibility of the map. For example, trypsin used as a cleavage agent will have to be treated with tosyl-l-phenylalanine chloromethyl ketone to inactivate chymotrypsin. Other methods, such as purification of trypsin by HPLC or immobilization of enzyme on a gel support, have been successfully used when only a small amount of protein is available.
Pretreatment of the Protein
Under certain conditions, it might be necessary to concentrate the sample, or to separate the protein from added substances and stabilizers used in the formulation of the product if these interfere with the mapping procedure. Physical procedures used for pretreatment can include ultrafiltration, column chromatography, and lyophilization.
Other pretreatments, such as the addition of chaotropic agents (e.g., urea) can be used to unfold the protein prior to mapping. To allow the enzyme to have full access to cleavage sites and permit some unfolding of the protein, it is often necessary to reduce and alkylate the disulfide bonds prior to digestion.
Digestion with trypsin can introduce ambiguities in the tryptic map as a result of side reactions occurring during the digestion reaction, such as nonspecific cleavage, deamidation, disulfide isomerization, oxidation of methionine residues, or formation of pyroglutamic groups created from the deamidation of glutamine at the N-terminal side of a peptide. Furthermore, peaks may be produced by autohydrolysis of trypsin. Their intensities depend on the ratio of trypsin to protein. To avoid autohydrolysis, solutions of proteases may be prepared at a pH that is not optimal (e.g., at pH 5 for trypsin), which would mean that the enzyme would not become active until diluted with the digest buffer.
Establishment of Optimal Digestion Conditions
Factors that affect the completeness and effectiveness of digestion of proteins are those that could affect any chemical or enzymatic reactions.
pH—
The digestion mixture pH is empirically determined to ensure the optimal performance of the given cleavage agent. For example, when using cyanogen bromide as a cleavage agent, a highly acidic environment (e.g., pH 2, formic acid) is necessary; however, when using trypsin as a cleavage agent, a slightly alkaline environment (pH 8) is optimal. As a general rule, the pH of the reaction milieu should not alter the chemical integrity of the protein during the digestion and should not change during the course of the fragmentation reaction.
Temperature—
A temperature between 25 and 37
and 37 is adequate for most digestions. The temperature used is intended to minimize chemical side reactions. The type of protein under test will dictate the temperature of the reaction milieu because some proteins are more susceptible to denaturation as the temperature of the reaction increases. For example, digestion of recombinant bovine somatropin is conducted at 4
is adequate for most digestions. The temperature used is intended to minimize chemical side reactions. The type of protein under test will dictate the temperature of the reaction milieu because some proteins are more susceptible to denaturation as the temperature of the reaction increases. For example, digestion of recombinant bovine somatropin is conducted at 4 because at higher temperatures it will precipitate during digestion.
because at higher temperatures it will precipitate during digestion.
Time—
If a sufficient amount of sample is available, a time course study is considered in order to determine the optimum time to obtain a reproducible map and avoid incomplete digestion. Time of digestion varies from 2 to 30 hours. The reaction is stopped by the addition of an acid that does not interfere with the tryptic map, or by freezing.
Amount of Cleavage Agent—
Although excessive amounts of cleavage agent are used to accomplish a reasonably rapid digestion time (i.e., 6 to 20 hours), the amount of cleavage agent is minimized to avoid its contribution to the chromatographic map pattern. A protein-to-protease ratio between 20:1 and 200:1 is generally used. It is recommended that the cleavage agent be added in two or more stages to optimize cleavage. Nonetheless, the final reaction volume remains small enough to facilitate the next step in peptide mapping—the separation step. To sort out digestion artifacts that might be interfering with the subsequent analysis, a blank determination is performed using a digestion control with all the reagents except the test protein.
Chromatographic Separation
Many techniques are used to separate peptides for mapping. The selection of a technique depends on the protein being mapped. Techniques that have been successfully used for the separation of peptides are shown in Table 2.
Table 2. Techniques Used for the Separation of Peptides
| Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) |
| Ion-Exchange Chromatography (IEC) |
| Hydrophobic Interaction Chromatography (HIC) |
| Polyacrylamide Gel Electrophoresis (PAGE), nondenaturating |
| Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) |
| Capillary Electrophoresis (CE) |
| Paper Chromatography—High Voltage (PCHV) |
| High-Voltage Paper Electrophoresis (HVPE) |
In this section, a most widely used reverse-phase HPLC (RP-HPLC) method is described as one of the procedures of chromatographic separation.
The purity of solvents and mobile phases is a critical factor in HPLC separation. HPLC-grade solvents and water that are commercially available are recommended for RP-HPLC. Dissolved gases present a problem in gradient systems where the solubility of the gas in a solvent may be less in a mixture than in a single solvent. Vacuum degassing and agitation by sonication are often used as useful degassing procedures. When solid particles in the solvents are drawn into the HPLC system, they can damage the sealing of pump valves or clog the top of the chromatographic column. Both pre- and post-pump filtration are also recommended.
Chromatographic Column—
The selection of a chromatographic column is empirically determined for each protein. Columns with 100 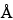 or 300
or 300 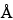 pore size and silica support can give optimal separation. For smaller peptides, octylsilane chemically bonded to totally porous silica particles 3 to 10 µm in diameter (L7) and of octadecylsilane chemically bonded to porous silica or ceramic microparticles 3 to 10 µm in diameter (L1) column packings are more efficient than the butyl silane chemically bonded to totally porous silica particles 5 to 10 µm in diameter (L26) packing.
pore size and silica support can give optimal separation. For smaller peptides, octylsilane chemically bonded to totally porous silica particles 3 to 10 µm in diameter (L7) and of octadecylsilane chemically bonded to porous silica or ceramic microparticles 3 to 10 µm in diameter (L1) column packings are more efficient than the butyl silane chemically bonded to totally porous silica particles 5 to 10 µm in diameter (L26) packing.
Solvent—
The most commonly used solvent is water with acetonitrile as the organic modifier to which less than 0.1% of trifluoroacetic acid is added. If necessary, add isopropyl alcohol or n-propyl alcohol to solubilize the digest components, provided that the addition does not unduly increase the viscosity of the components.
Mobile Phase—
Buffered mobile phases containing phosphate are used to provide some flexibility in the selection of pH conditions, because shifts of pH in the 3.0 to 5.0 range enhance the separation of peptides containing acidic residues (e.g., glutamic and aspartic acids). Sodium or potassium phosphates, ammonium acetate, phosphoric acid, and a pH between 2 and 7 (or higher for polymer-based supports) have also been used with acetonitrile gradients. Acetonitrile-containing trifluoroacetic acid is also used quite often.
Gradient Selection—
Gradients can be linear, nonlinear, or include step functions. A shallow gradient is recommended in order to separate complex mixtures. Gradients are optimized to provide clear resolution of one or two peaks that will become “marker” peaks for the test.
Isocratic Selection—
Isocratic HPLC systems using a single mobile phase are used on the basis of their convenience of use and improved detector responses. Optimal composition of a mobile phase to obtain clear resolution of each peak is sometimes difficult to establish. Mobile phases for which slight changes in component ratios or in pH significantly affect retention times of peaks in peptide maps should not be used in isocratic HPLC systems.
Other Parameters—
Temperature control of the column is usually necessary to achieve good reproducibility. The flow rates for the mobile phases range from 0.1 to 2.0 mL per minute, and the detection of peptides is performed with a UV detector at 200 to 230 nm. Other methods of detection have been used (e.g., postcolumn derivatization), but they are not as robust or as versatile as UV detection.
System Suitability—
The section System Suitability under Chromatography  621
621 provides an experimental means for measuring the overall performance of the test method. The acceptance criteria for system suitability depend on the identification of critical test parameters that affect data interpretation and acceptance. These critical parameters are also criteria that monitor peptide digestion and peptide analysis. An indicator that the desired digestion endpoint was achieved is the comparison with a Reference Standard or Reference Material, which is treated exactly as the article under test. The use of a USP Reference Standard in parallel with the protein under test is critical in the development and establishment of system suitability limits. In addition, a specimen chromatogram should be included with the USP Reference Standard or Reference Material for additional comparison purposes. Other indicators may include visual inspection of protein or peptide solubility, the absence of intact protein, or measurement of responses of a digestion-dependent peptide. The critical system suitability parameters for peptide analysis will depend on the particular mode of peptide separation and detection, and on the data analysis requirements.
provides an experimental means for measuring the overall performance of the test method. The acceptance criteria for system suitability depend on the identification of critical test parameters that affect data interpretation and acceptance. These critical parameters are also criteria that monitor peptide digestion and peptide analysis. An indicator that the desired digestion endpoint was achieved is the comparison with a Reference Standard or Reference Material, which is treated exactly as the article under test. The use of a USP Reference Standard in parallel with the protein under test is critical in the development and establishment of system suitability limits. In addition, a specimen chromatogram should be included with the USP Reference Standard or Reference Material for additional comparison purposes. Other indicators may include visual inspection of protein or peptide solubility, the absence of intact protein, or measurement of responses of a digestion-dependent peptide. The critical system suitability parameters for peptide analysis will depend on the particular mode of peptide separation and detection, and on the data analysis requirements.
When peptide mapping is used as an identification test, the system suitability requirements for the identified peptides cover selectivity and precision. In this case, as well as when identification of variant proteins is done, the identification of the primary structure of the peptide fragments in the peptide map provides both a verification of the known primary structure and the identification of protein variants by comparison with the peptide map of the USP Reference Standard or Reference Material for the specified protein. The use of a digested USP Reference Standard or Reference Material for a given protein in the determination of peptide resolution is the method of choice. For an analysis of a variant protein, a characterized mixture of a variant and a Reference Standard can be used, especially if the variant peptide is located in a less-resolved region of the map. The index of pattern consistency can be simply the number of major peptides detected. Peptide pattern consistency can be best defined by the resolution of peptide peaks. Chromatographic parameters—such as peak-to-peak resolution, maximum peak width, peak area, peak tailing factors, and column efficiency—may be used to define peptide resolution. Depending on the protein under test and the method of separation used, single peptide or multiple peptide resolution requirements may be necessary.
The replicate analysis of the digest of the USP Reference Standard or Reference Material for the protein under test yields measures of precision and quantitative recovery. Recovery of the identified peptides is generally ascertained by the use of internal or external peptide standards. The precision is expressed as the relative standard deviation (RSD). Differences in the recovery and precision of the identified peptides are expected; therefore, the system suitability limits will have to be established for both the recovery and the precision of the identified peptides. These limits are unique for a given protein and will be specified in the individual monograph.
Visual comparison of the relative retention times, the peak responses (the peak area or the peak height), the number of peaks, and the overall elution pattern is completed initially. It is then complemented and supported by mathematical analysis of the peak response ratios and by the chromatographic profile of a 1:1 (v/v) mixture of sample and USP Reference Standard or Reference Material digest. If all peaks in the sample digest and in the USP Reference Standard or Reference Material digest have the same relative retention times and peak response ratios, then the identity of the sample under test is confirmed.
If peaks that initially eluted with significantly different relative retention times are then observed as single peaks in the 1:1 mixture, the initial difference would be an indication of system variability. However, if separate peaks are observed in the 1:1 mixture, this would be evidence of the nonequivalence of the peptides in each peak. If a peak in the 1:1 mixture is significantly broader than the corresponding peak in the sample and USP Reference Standard or Reference Material digest, it may indicate the presence of different peptides. The use of computer-aided pattern recognition software for the analysis of peptide mapping data has been proposed and applied, but issues related to the validation of the computer software preclude its use in a compendial test in the near future. Other automated approaches have been used that employ mathematical formulas, models, and pattern recognition. Such approaches, for example the automated identification of compounds by IR spectroscopy and the application of diode-array UV spectral analysis for identification of peptides, have been proposed. These methods have limitations due to inadequate resolutions, co-elution of fragments, or absolute peak response differences between USP Reference Standard or Reference Material and sample fragments.
The numerical comparison of the retention times and peak areas or peak heights can be done for a selected group of relevant peaks that have been correctly identified in the peptide maps. Peak areas can be calculated using one peak showing relatively small variation as an internal reference, keeping in mind that peak area integration is sensitive to baseline variation and is likely to introduce error into the analysis. Alternatively, the percentage of each peptide peak height relative to the sum of all peak heights can be calculated for the sample under test. The percentage is then compared to that of the corresponding peak of the USP Reference Standard or Reference Material. The possibility of autohydrolysis of trypsin is monitored by producing a blank peptide map, that is, the peptide map obtained when a blank solution is treated with trypsin.
The minimum requirement for the qualification of peptide mapping is an approved test procedure that includes system suitability as a test control. In general, early in the regulatory process, qualification of peptide mapping for a protein is sufficient. As the regulatory approval process for the protein progresses, additional qualifications of the test can include a partial validation of the analytical procedure to provide assurance that the method will perform as intended in the development of a peptide map for the specified protein.
Analysis and Identification of Peptides
This section gives guidance on the use of peptide mapping during development in support of regulatory applications.
The use of a peptide map as a qualitative tool does not require the complete characterization of the individual peptide peaks. However, validation of peptide mapping in support of regulatory applications requires rigorous characterization of each of the individual peaks in the peptide map. Methods to characterize peaks range from N-terminal sequencing of each peak followed by amino acid analysis to the use of mass spectroscopy (MS).
For characterization purposes, when N-terminal sequencing and amino acid analysis are used, the analytical separation is scaled up. Because scale-up might affect the resolution of peptide peaks, it is necessary, using empirical data, to ensure that there is no loss of resolution due to scale-up. Eluates corresponding to specific peptide peaks are collected, vacuum-concentrated, and chromatographed again, if necessary. Amino acid analysis of fragments may be limited by the peptide size. If the N-terminus is blocked, it may need to be cleared before sequencing. C-terminal sequencing of proteins in combination with carboxypeptidase digestion and matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) MS can also be used for characterization purposes.
The use of MS for characterization of peptide fragments is by direct infusion of isolated peptides or by the use of on-line LC-MS for structure analysis. In general, it includes electrospray and MALDI-TOF analyzers as well as fast atom bombardment (FAB). Tandem MS has also been used to sequence a modified protein and to determine the type of amino acid modification that has occurred. The comparison of mass spectra of the digests before and after reduction provides a method to assign the disulfide bonds to the various sulfhydryl-containing peptides.
If regions of the primary structure are not clearly demonstrated by the peptide map, it might be necessary to develop a secondary peptide map. The goal of a validated method of characterization of a protein through peptide mapping is to reconcile and account for at least 95% of the theoretical composition of the protein structure.
A validated peptide map can be used to assess the integrity of the predicted primary sequence of a protein product (i.e., its genetic stability). It can also be used to determine lot-to-lot consistency of the biotechnology-derived product process. Furthermore, the performance of the protein expression of the production system is best assessed by peptide mapping of the expressed protein. Peptide maps of protein produced at various times of the protein expression process, including a point well beyond the normal protein expression time, compared with those of a USP Reference Standard or Reference Material, will evaluate the genetic stability of the expression system as a function of time.
Variant protein sequences can arise from a genetic variation at the DNA level (point mutation) or as an error in the translation process. A validated peptide map is the best approach to the detection of protein variants. However, the limitations of the peptide mapping itself must be taken into consideration. The detection of a structured variant is possible only if the corresponding peptide variant is easily isolated and characterized. To establish genetic stability will require the use of a battery of biochemical methods, provided that the variants have properties different from those of the “normal” protein.
Critical Factors
Validation of peptide mapping requires that a protocol be designed, outlining in detail the experiment to be conducted and the criteria for acceptance of the map. Criteria for acceptance of mapping include detection limit, specificity, linearity, range, accuracy, precision, and reagent stability. Reproducibility of the peptide map is a critical element in the utilization of such a map as an identity test and for confirming genetic stability. Those technical aspects of peptide mapping that influence the reproducibility of the map will be discussed.
The setting of limits, with respect to quantification (peak area or height) and identification (retention times) for the selected group of relevant peaks is based on empirical observations. These limits detect significant differences between the sample and USP Reference Standard or Reference Material within a series of analyses.
Another critical issue is the recovery of peptides and its impact on peak area determination and reproducibility and on the establishment of acceptance criteria. The recovery criteria address all aspects of test methodology, from digestion to chromatographic conditions. Determination of peptide recovery includes quantitative amino acid analysis, spike addition, radiolabeling, and UV summation. An overall recovery of about 80% is considered satisfactory. Recovery of individual peptides is more problematic and is handled on a case-by-case basis. The critical factors considered in the validation of a peptide map are as follows.
Written Test Procedures—
These procedures include a detailed description of the analytical method in which reagents, equipment, sample preparation, method of analysis, and analysis of the data are defined.
Validation Protocol—
A protocol is prepared that contains a procedure for test validation.
Acceptance Criteria—
The criteria can be minimal at the early stages, but need to be better defined as validation studies progress.
Reporting of Results—
Results from the validation study are documented with respect to the analytical parameters listed in the validation protocol.
Revalidation of the Test Procedure—
If the method used requires alteration that could affect the analytical parameter previously assessed in the validation of the procedure, the test procedure must be revalidated. Significant changes in the processing of the article, in laboratories performing the analysis, in formulation of the bulk or the finished products, and in any other significant parameter will require revalidation of the methods.
Requirements
Precision
Intratest Precision—
This is a measure of the reproducibility of peptide mapping. The two critical steps in peptide mapping are fragmentation (i.e., digestion) and separation of peptides. An acceptable precision occurs where the absolute retention times and the relative peak areas are constant from run to run, and the average variation in retention time is small relative to that of a selected internal reference peak. The reproducibility of the map can be enhanced if a temperature-controlled column oven is used, if an extensive equilibration of the system is performed prior to the start of the test, if a blank (control digest mixture without protein) is run first to minimize “first run effects,” and if a USP Reference Standard or a Reference Material digest is interspersed periodically with test samples to evaluate chromatographic drift.
The criteria for validation of the fragmentation step are similar to those described below for separation of peptides, but they are met for consecutive tests of a series of separately prepared digests of the protein under test.
The criteria for validation of the separation of peptides step include the following:
- The average standard deviation of the absolute retention times of all major peaks for a set of consecutive tests of the same digest does not exceed a specified acceptance criterion.
- The average standard deviation of absolute peak area for all fully resolved major peaks does not exceed a specified percentage.
Intertest Precision—
This is a measure of the reproducibility of the peptide mapping when the test is performed on different days, by different analysts, in different laboratories, with reagents or enzymes from different suppliers or different lots from the same supplier, with different instruments, on columns of different makes or columns of the same make from different lots, and on individual columns of the same make from the same lot. Although it would be desirable, from a scientific perspective, to validate all of these variables in terms of their impacts on precision, a practical approach is to validate the test using those variables most likely to be encountered under operational conditions. Additional variables can be included when needed.
The experimental design allows the analyst to make comparisons using peak retention times and areas that are expressed relative to a highly reproducible internal reference peak within the same chromatogram. The relative peak area is expressed as the ratio of the peak area to that of the internal reference peak. The relative retention time can be expressed as the difference between the absolute retention time and that of the reference peak. The use of relative values eliminates the need to make separate corrections for differences due to injector-to-injector volumes, units of measure for peak areas, column dimensions, and instrument dead volumes. The variability in the retention times and peak areas for the Intertest Precision experiments is expected to be slightly higher than the variability observed for Intratest Precision.
Robustness
Factors such as composition of the Mobile Phase, protease quality or chemical reagent purity, column variation and age, and digest stability are likely to affect the overall performance of the test and its reproducibility. Tolerances for each of the key parameters are evaluated and baseline limits established in case the test is used for routine lot release purposes.
Mobile Phase—
The composition of the Mobile Phase is optimized to obtain the maximum resolution of peptides throughout the elution profile. A balance between optimal resolution and overall reproducibility is desired. A lower pH might improve peak separation but might shorten the life of the column, resulting in lack of reproducibility. Peptide maps at a pH above and below the pH of the procedure are compared to the peptide map obtained at the pH of the procedure and checked for significant differences; they are also reviewed with respect to the acceptance criteria established in the validation protocol.
Protease Quality or Chemical Reagent Purity—
A sample of the USP Reference Standard or Reference Material for the protein under test is prepared and digested with different lots of cleavage agent. The chromatograms for each digest are compared in terms of peak areas, shape, and number. The same procedure can be applied to other critical chemicals or pretreatment procedures used during sample preparation, such as reducing and carboxymethylation reagents.
Column Considerations—
Column-to-column variability, even within a single lot, can affect the performance of the column in the development of peptide maps. Column size may also lead to significant differences. A USP Reference Standard or Reference Material of the protein under test is digested and the digest is chromatographed on different lots of column from a single manufacturer. The maps are then evaluated in terms of the overall elution profile, retention times, selectivity resolution, and recovery. To evaluate the overall lifetime of the column in terms of robustness, perform a peptide mapping test on different columns and vary significantly the number of injections (e.g., from 10 injections to 250 injections). The resulting maps can then be compared for significant differences in peak broadening, peak area, and overall resolution. As a column ages, an increase in back pressure might be observed that might affect the peptide maps.
A sensible precaution in the use of peptide mapping columns is to select alternative columns in case the original columns become unavailable or are discontinued. Perform a peptide mapping test using equivalent columns from different manufacturers, and examine the maps. Differences in particle shape and size, pore size and volume, carbon load, and end-capping can lead to significant differences in retention times, elution profile selectivity, resolution, and recovery. Slight modifications in the gradient profile may be required to achieve equivalency of mapping when using columns from different manufacturers. [note—The equivalency between instrumentation used for the validation of the test and for routine quality control testing should be considered. It might be preferable to use the same HPLC system for all applications. Otherwise, equivalency of the systems is determined, which may require some changes in the chromatographic test conditions. ]
Digest Stability—
The length of time a digest can be kept before it is chromatographed, as well as the conditions under which the digest is stored before chromatography, is assessed. Several aliquots from a single digest are stored at different storage conditions and chromatographed. These maps are then evaluated for significant differences.
Reproducibility
Determination of various parameters indicated above is repeated using the same USP Reference Standard or Reference Material and test sample in at least two different laboratories by two analysts equipped with similar HPLC systems. The generated peptide maps are evaluated for significant differences.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Maura C Kibbey, Ph.D.
Senior Scientific Liaison, Biologics & Biotechnology (301) 230-6309 |
(GCBA2010) General Chapters - Biological Analysis |
USP38–NF33 Page 954
Pharmacopeial Forum: Volume No. 40(3)



