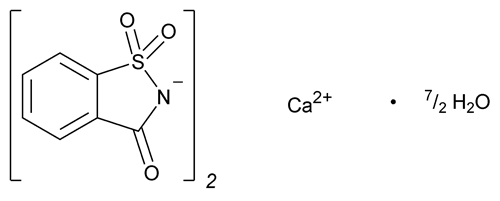
Saccharin Calcium
C14H8CaN2O6S2404.44
1,2-Benzisothiazol-3(2H)-one, 1,1-dioxide, calcium salt, hydrate (2:7);
1,2-Benzisothiazolin-3-one 1,1-dioxide calcium salt hydrate (2:7)

 [6381-91-5].
[6381-91-5].
Anhydrous

 [6485-34-3].
[6485-34-3].
(sak' a rin kal' see um).
C14H8CaN2O6S2404.44
1,2-Benzisothiazol-3(2H)-one, 1,1-dioxide, calcium salt, hydrate (2:7);
1,2-Benzisothiazolin-3-one 1,1-dioxide calcium salt hydrate (2:7)
Anhydrous
DEFINITION
Saccharin Calcium contains NLT 99.0% and NMT 101.0% of C14H8CaN2O6S2, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
Sample: Dry at 105 to constant weight.
to constant weight.
• B. Procedure
Sample solution: 100 mg/mL
Analysis: To the Sample solution add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide. Add 3 N hydrochloric acid, dropwise, until the solution is acid to the indicator. Add ammonium oxalate TS.
Acceptance criteria: A white precipitate is formed when the ammonium oxalate is added. This precipitate is insoluble in 6 N acetic acid but dissolves in hydrochloric acid.
• C. Calcium salts moistened with hydrochloric acid impart a transient yellowish-red color to a nonluminous flame.
ASSAY
• Procedure
Sample solution: 3 mg/mL of Saccharin Calcium in glacial acetic acid. [Note—Slight heating may be needed to dissolve the sample. ]
Analysis: Titrate Sample solution with 0.1 N perchloric acid, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 20.22 mg of C14H8CaN2O6S2.
). Each mL of 0.1 N perchloric acid is equivalent to 20.22 mg of C14H8CaN2O6S2.
Acceptance criteria: 99.0%–101.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method I  231
231
Sample: 4 g in 46 mL
Analysis: Add 4 mL of dilute hydrochloric acid (1 in 12), mix, and rub the inner wall of the vessel with a glass rod until crystallization begins. Allow the solution to stand for 1 h, then pass through a dry filter, discarding the first 10 mL of the filtrate, and use 25 mL of the subsequent filtrate for the Sample preparation.
Acceptance criteria: NMT 10 ppm
Organic Impurities
• Procedure 1: Limit of Toluenesulfonamides
Internal standard solution: 0.25 mg/mL of caffeine in methylene chloride
Standard stock solution: 20.0 µg/mL of USP o-Toluenesulfonamide RS and 20.0 µg/mL of USP p-Toluenesulfonamide RS in methylene chloride
Standard solution: Evaporate 5.0 mL of Standard stock solution to dryness in a stream of nitrogen. Dissolve the residue in 1.0 mL of the Internal standard solution.
Sample stock solution: 200 mg/mL in water. If necessary, adjust with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of 7–8 before final dilution.
Sample solution: Shake 50 mL of the Sample stock solution with four quantities each of 50 mL of methylene chloride. Combine the lower layers, dry over anhydrous sodium sulfate, and filter. Wash the filter and the sodium sulfate with 10 mL of methylene chloride. Combine the solution and the washings, and evaporate almost to dryness in a water bath at a temperature not exceeding 40 . Using a small quantity of methylene chloride, quantitatively transfer the residue into a suitable 10-mL tube, evaporate to dryness in a stream of nitrogen, and dissolve the residue in 1.0 mL of the Internal standard solution.
. Using a small quantity of methylene chloride, quantitatively transfer the residue into a suitable 10-mL tube, evaporate to dryness in a stream of nitrogen, and dissolve the residue in 1.0 mL of the Internal standard solution.
Blank solution: Evaporate 200 mL of methylene chloride to dryness in a water bath at a temperature not exceeding 40 . Dissolve the residue in 1 mL of methylene chloride.
. Dissolve the residue in 1 mL of methylene chloride.
Chromatographic system
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 10-m fused silica column, coated with G3 phase (film thickness 2 µm)
Temperature
Injector: 250
Detector: 250
Column: 180
Carrier gas: Nitrogen
Flow rate: 10 mL/min
Injection size: 1 µL
Split ratio: 2:1
System suitability
Samples: Standard solution and Blank solution
[Note—The substances are eluted in the following order: o-toluenesulfonamide, p-toluenesulfonamide, and caffeine. ]
Suitability requirements: There are no peaks present in the Blank solution at the retention times of the Internal standard solution, o-toluenesulfonamide, and p-toluenesulfonamide, Blank solution.
Resolution: NLT 1.5 between o-toluenesulfonamide and p-toluenesulfonamide, Standard solution
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: If any peaks due to o-toluenesulfonamide and p-toluenesulfonamide appear in the chromatogram obtained with the Sample solution, the ratio of their areas to that of the Internal standard solution is NMT the corresponding ratio in the chromatogram obtained with the Standard solution.
Individual impurities: See Impurity Table 1.
Impurity Table 1
| Name | Acceptance Criteria NMT (ppm) |
|---|---|
| o-Toluenesulfonamide | 10 |
| p-Toluenesulfonamide | 10 |
• Procedure 2: Limit of Benzoate and Salicylate
Sample solution: 50 mg/mL
Analysis: To 10 mL of the Sample solution add 5 drops of 6 N acetic acid and 3 drops of ferric chloride TS.
Acceptance criteria: No precipitate or violet color appears.
SPECIFIC TESTS
• Water Determination, Method I  921
921 : NMT 15.0%
: NMT 15.0%
• Readily Carbonizable Substances  271
271
Sample solution: 40 mg/mL in sulfuric acid (94.5%–95.5% (w/w) of H2SO4), 48 –50
–50 for 10 min
for 10 min
Acceptance criteria: The Sample solution has no more color than Matching Fluid A, when viewed against a white background.
• Clarity of Solution
[Note—The Sample solution is to be compared to Reference suspension A and to water in diffused daylight 5 min after preparation of Reference suspension A. ]
Diluent: 200 g/L solution of sodium acetate
Hydrazine solution: 10.0 mg/mL of hydrazine sulfate. [Note—Allow to stand for 4–6 h. ]
Methenamine solution: Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25.0 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension: Transfer 25.0 mL of Hydrazine solution to the Methenamine solution in the 100-mL glass-stoppered flask. Mix, and allow to stand for 24 h. [Note—This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use. ]
Opalescence standard: Transfer 15.0 mL of the Primary opalescent suspension and dilute to 1000 mL. [Note—This suspension should not be used beyond 24 h after preparation. ]
Reference suspension A: Opalescence standard and water (1 in 20)
Reference suspension B: Opalescence standard and water (1 in 10)
Sample solution: 200 mg/mL in Diluent
Analysis
Samples: Diluent, Reference suspension A, Reference suspension B, Sample solution, and water
Transfer a sufficient portion of Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Reference suspension A, Reference suspension B, water, and Diluent to separate matching test tubes. Compare solutions in diffused daylight, viewing vertically against a black background (see Spectrophotometry and Light-Scattering  851
851 , Visual Comparison). [Note—The diffusion of light must be such that Reference suspension A can readily be distinguished from water, and that Reference suspension B can readily be distinguished from Reference suspension A. ]
, Visual Comparison). [Note—The diffusion of light must be such that Reference suspension A can readily be distinguished from water, and that Reference suspension B can readily be distinguished from Reference suspension A. ]
Acceptance criteria: The Sample solution shows the same clarity as that of water, or Diluent, or its opalescence is NMT that of Reference suspension A.
• Color of Solution
Diluent A: 200 g/L solution of sodium acetate
Diluent B: 10 g/L solution of hydrochloric acid
Standard stock solution: Ferric chloride CS, cobaltous chloride CS, cupric sulfate CS, and Diluent B (3.0:3.0:2.4:1.6)
Standard solution: Standard stock solution and Diluent B (1:99). [Note—Prepare the Standard solution immediately before use. ]
Sample solution: Use the Sample solution from Clarity of Solution.
Analysis
Samples: Diluent A, Standard solution, Sample solution, and water
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of the Standard solution, Diluent A, and water to separate matching test tubes. Compare solutions in diffused daylight, viewing vertically against a white background (see Spectrophotometry and Light-Scattering  851
851 , Visual Comparison).
, Visual Comparison).
Acceptance criteria: The Sample solution has the appearance of water or Diluent A, or is not more intensely colored than the Standard solution.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
• Labeling: Where the quantity of saccharin calcium is indicated in the labeling of any preparation containing Saccharin Calcium, this shall be expressed in terms of saccharin (C7H5NO3S).
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S. Scientific Liaison 1-301-816-8335 | (EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4596
Pharmacopeial Forum: Volume No. 32(4) Page 1114Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.