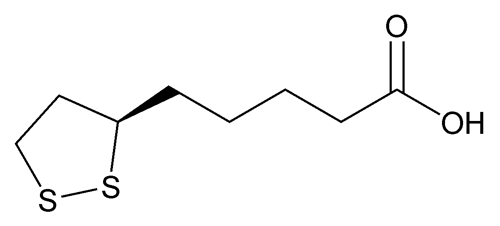
Alpha Lipoic Acid
C8H14O2S2206.33
Thioctic acid;
1,2-Dithiolane-3-pentanoic acid;
1,2-Dithiolane-3-valeric acid

 [1077-28-7].
[1077-28-7]. • A.
• A. USP35 The retention time of the peak for alpha lipoic acid of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
USP35 The retention time of the peak for alpha lipoic acid of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. • B. Infrared Absorption
• B. Infrared Absorption  197K
197K
 USP35
USP35 Buffer solution:
Buffer solution: USP35 0.68 g/L of monobasic potassium phosphate
USP35 0.68 g/L of monobasic potassium phosphate
 USP35
USP35
 USP35
USP35 Diluted standard solution: Dilute the Standard solution (1 in 1000) with Mobile phase.
Diluted standard solution: Dilute the Standard solution (1 in 1000) with Mobile phase.  USP35
USP35
C8H14O2S2206.33
Thioctic acid;
1,2-Dithiolane-3-pentanoic acid;
1,2-Dithiolane-3-valeric acid
DEFINITION
Alpha Lipoic Acid contains NLT 99.0% and NMT 101.0% of C8H14O2S2, calculated on the dried basis.
IDENTIFICATION
Change to read:
Add the following:
ASSAY
Change to read:
• Procedure Mobile phase: Methanol, Buffer solution, and acetonitrile (58:46:9). Adjust with phosphoric acid solution (8.3 in 100) to a pH of 3.0–3.1.
Standard solution: 1.0 mg/mL of USP Alpha Lipoic Acid RS in  Mobile phase
Mobile phase USP35
USP35
Sample solution: 1.0 mg/mL of Alpha Lipoic Acid in  Mobile phase
Mobile phase USP35
USP35
Chromatographic system
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 250-mm; packing L1
Column temperature: 35
Flow rate: 1.2 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT  10,000
10,000 USP35 theoretical plates
USP35 theoretical plates
Tailing factor: NMT 2.0 for the alpha lipoic acid peak
Relative standard deviation: NMT 2.0% for alpha lipoic acid
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of alpha lipoic acid (C8H14O2S2) in the portion of Alpha Lipoic Acid taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Alpha Lipoic Acid RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Alpha Lipoic Acid in the Sample solution (mg/mL) |
Acceptance criteria: 99.0%–101.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 : Less than 0.1%
: Less than 0.1%
• Heavy Metals, Method II  231
231 : NMT 10 ppm
: NMT 10 ppm
Change to read:
• Chromatographic Purity, Procedure 1 Buffer solution, Mobile phase, Standard solution, 
 USP35 Sample solution, and Chromatographic system: Proceed as directed in the Assay.
USP35 Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability
Sample: Diluted standard solution
Suitability requirements
Signal-to-noise ratio: NLT 10
Relative standard deviation: NMT 10.0%
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Alpha Lipoic Acid taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rT | = | = sum of the responses of all the peaks from the Sample solution |
Acceptance criteria
Individual impurities: NMT 0.1%
Total impurities: NMT 2.0%
• Chromatographic Purity, Procedure 2
[Note—Use low-actinic glassware. ]
Standard solution A: 40.0 mg/mL of USP Alpha Lipoic Acid RS in dimethylformamide
Standard solution B: 20.0 mg/mL of USP Alpha Lipoic Acid RS in dimethylformamide, prepared from the dilution of Standard solution A
Standard solution C: 10.0 mg/mL of USP Alpha Lipoic Acid RS in dimethylformamide, prepared from the dilution of Standard solution B
Sample solution: 40.0 mg/mL of Alpha Lipoic Acid in dimethylformamide
Chromatographic system
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 5 µL
Developing solvent system: n-Propyl alcohol, ethyl acetate, water, and 25% ammonia water (40:40:10:5). Allow the chamber to become saturated for at least 1 h.
Iodine vapor–saturated chamber: Transfer 4 g of iodine crystals to a small watch glass, and place in a chromatographic chamber. Allow the chamber to become saturated for at least 2 h.
Analysis
Samples: Standard solution A, Standard solution B, Standard solution C, and Sample solution
Proceed as directed in the chapter, except to develop until the solvent front has moved 10 cm. Remove the plate, and allow to air-dry until the ammonia disappears completely. Heat at 50 for 20 min, cool the plate, and place in the Iodine vapor–saturated chamber until the spots are visible. The RF value for the alpha lipoic acid spot is 0.25–0.30 and for the polymeric lipoic acid spot is 0.
for 20 min, cool the plate, and place in the Iodine vapor–saturated chamber until the spots are visible. The RF value for the alpha lipoic acid spot is 0.25–0.30 and for the polymeric lipoic acid spot is 0.
Acceptance criteria: No spot other than the alpha lipoic acid spot from the Sample solution is more intense than the spot at RF = 0 from Standard solution A.
SPECIFIC TESTS
• Melting Range or Temperature  741
741 : 60.0
: 60.0 –62.0
–62.0
• Optical Rotation, Specific Rotation  781S
781S
Sample solution: 50 mg/mL of Alpha Lipoic Acid, in dehydrated alcohol
Acceptance criteria: –1.0 to +1.0
to +1.0
• Loss on Drying  731
731 : Dry a sample in vacuum at 40
: Dry a sample in vacuum at 40 for 3 h: it loses NMT 0.2% of its weight.
for 3 h: it loses NMT 0.2% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S. Scientific Liaison 1-301-816-8594 | (DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1366
Pharmacopeial Forum: Volume No. 37(1)Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.