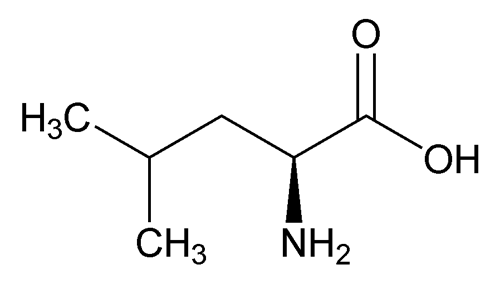
Leucine
(loo' seen).
DEFINITION
Leucine contains NLT 98.5% and NMT 101.5% of l-leucine (C6H13NO2), calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Sample: 130 mg of Leucine
Blank: Mix 3 mL of formic acid and 50 mL of glacial acetic acid.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 3 mL of formic acid and 50 mL of glacial acetic acid. Titrate with the Titrant. Perform the Blank determination.
Calculate the percentage of leucine (C6H13NO2) in the Sample taken:
Result = {[(VS  VB) × N × F]/W} × 100
VB) × N × F]/W} × 100
| VS | = | = Titrant volume consumed by the Sample (mL) |
| VB | = | = Titrant volume consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 131.2 mg/mEq |
| W | = | = Sample weight (mg) |
Acceptance criteria: 98.5%–101.5% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 : NMT 0.4%
: NMT 0.4%
• Chloride and Sulfate, Chloride  221
221
Standard solution: 0.50 mL of 0.020 N hydrochloric acid
Sample: 0.73 g of Leucine
Acceptance criteria: NMT 0.05%
• Chloride and Sulfate, Sulfate  221
221
Standard solution: 0.10 mL of 0.020 N sulfuric acid
Sample: 0.33 g of Leucine
Acceptance criteria: NMT 0.03%
• Iron  241
241 : NMT 30 ppm
: NMT 30 ppm
• Heavy Metals, Method II  231
231 : NMT 15 ppm
: NMT 15 ppm
• Related Compounds
System suitability solution: 0.4 mg/mL each of USP l-Leucine RS and USP l-Valine RS in 0.1 N hydrochloric acid
Standard solution: 0.05 mg/mL of USP l-Leucine RS in 0.1 N hydrochloric acid. [Note—This solution has a concentration equivalent to 0.5% of that of the Sample solution. ]
Sample solution: 10 mg/mL of Leucine in 0.1 N hydrochloric acid
Chromatographic system
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 5 µL
Developing solvent system: Butyl alcohol, glacial acetic acid, and water (3:1:1)
Spray reagent: 2 mg/mL of ninhydrin in a mixture of butyl alcohol and 2 N acetic acid (95:5)
System suitability
Suitability requirements: The chromatogram of the System suitability solution exhibits two clearly separated spots.
Analysis
Samples: System suitability solution, Standard solution, and Sample solution
After air-drying the plate, spray with Spray reagent, and heat between 100 and 105
and 105 for 15 min. Examine the plate under white light.
for 15 min. Examine the plate under white light.
Acceptance criteria: Any secondary spot of the Sample solution is not larger or more intense than the principal spot of the Standard solution.
Individual impurities: NMT 0.5%
Total impurities: NMT 2.0%
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution: 40 mg/mL in 6 N hydrochloric acid
Acceptance criteria: +14.9 to +17.3
to +17.3
• Loss on Drying  731
731 : Dry a sample at 105
: Dry a sample at 105 for 3 h: it loses NMT 0.2% of its weight.
for 3 h: it loses NMT 0.2% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S. Scientific Liaison 1-301-816-8594 | (DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3649
Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.