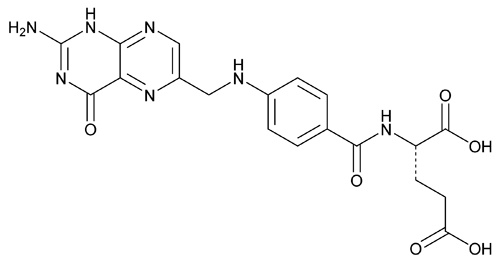
Folic Acid
C19H19N7O6441.40
l-Glutamic acid, N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-;
N-[p-[[(2-Amino-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]-l-glutamic acid

 [59-30-3].
[59-30-3].
(foe' lik as' id).
C19H19N7O6441.40
l-Glutamic acid, N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-;
N-[p-[[(2-Amino-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]-l-glutamic acid
DEFINITION
Folic Acid contains NLT 97.0% and NMT 102.0% of folic acid (C19H19N7O6), calculated on the anhydrous basis.
IDENTIFICATION
• A. Ultraviolet Absorption  197U
197U
Sample solution: 10 µg/mL in 0.1 N sodium hydroxide solution
Acceptance criteria: Meets the requirements. The ratio A256/A365 is 2.80–3.00.
ASSAY
• Procedure
[Note—Use low-actinic glassware throughout the following procedure. ]
3 N phosphoric acid: 98 g/L of phosphoric acid in water
6 N ammonium hydroxide: Dilute 40 mL of ammonium hydroxide with water to 100 mL.
Mobile phase: Transfer 2.0 g of monobasic potassium phosphate into a 1000-mL volumetric flask, and dissolve in 650 mL of water. Add 15.0 mL of a solution of 0.5 M tetrabutylammonium hydroxide in methanol, 7.0 mL of 3 N phosphoric acid, and 270 mL of methanol. Cool to room temperature, adjust with 3 N phosphoric acid or 6 N ammonium hydroxide to a pH of 5.0, and dilute with water to volume. Recheck the pH before use.
Internal standard solution: 2 mg/mL of methylparaben in Mobile phase. Dissolve the methylparaben first with methanol (about 4% of the final volume), and dilute with Mobile phase to volume.
Standard stock solution: 1 mg/mL of USP Folic Acid RS in Mobile phase. Dissolve the folic acid with the aid of 10% ammonium hydroxide (about 1% of the final volume), and dilute with Mobile phase to volume.
Standard solution: Transfer 4.0 mL of Standard stock solution and 4.0 mL of Internal standard solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Sample stock solution: Transfer 100 mg of Folic Acid to a 100-mL volumetric flask, and dissolve in 40 mL of Mobile phase and 1 mL of 10% ammonium hydroxide. Dilute with Mobile phase to volume.
Sample solution: Transfer 4.0 mL of Sample stock solution and 4.0 mL of Internal standard solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Chromatographic system
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm × 25-cm; packing L1
Flow rate: 1.2 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 3.6 between methylparaben and folic acid
Relative standard deviation: NMT 2.0% for the ratios of the folic acid peak area to the internal standard peak area
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of folic acid (C19H19N7O6) in the sample taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = internal standard ratio (peak response of folic acid/peak response of the internal standard) from the Sample solution |
| RS | = | = internal standard ratio (peak response of folic acid/peak response of the internal standard) from the Standard solution |
| CS | = | = concentration of USP Folic Acid RS in the Standard stock solution (mg/mL) |
| CU | = | = concentration of Folic Acid in the Sample stock solution (mg/mL) |
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 : NMT 0.3%
: NMT 0.3%
• Related Compounds
3 N phosphoric acid, 6 N ammonium hydroxide, Internal standard solution, Standard stock solution, Standard solution, and Chromatographic system: Proceed as directed in the Assay.
Sample solution: Use the Sample stock solution, prepared as directed in the Assay.
Analysis
Sample: Sample solution
Allow the Sample solution to elute for NLT 2 times the retention time of folic acid. Record the chromatogram, and measure the areas of all the peaks.
Calculate the percentage of total secondary peaks in the portion of Folic Acid taken:
Result = (rU/rT) × 100
| rU | = | = sum of the areas of all the peaks except that of the folic acid peak |
| rT | = | = sum of the areas of all the peaks |
Acceptance criteria: NMT 2.0%
SPECIFIC TESTS
• Water Determination, Method I  921
921
Analysis: Proceed as directed in the chapter, except stir the methanol solvent before and during the addition of the test specimen and during the titration.
Acceptance criteria: NMT 8.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed, light-resistant containers.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S. Scientific Liaison 1-301-816-8594 | (DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3275
Pharmacopeial Forum: Volume No. 29(2) Page 409Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.