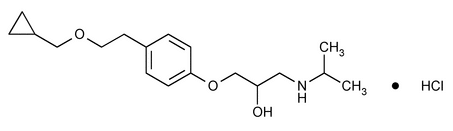
Betaxolol Hydrochloride
(be tax' oh lol hye'' droe klor' ide).
2-Propanol, 1-4-2-(cyclopropylmethoxy)ethylphenoxy-3-(1-methylethyl)amino-, hydrochloride, (±)-.
(±)-1-p-2-(Cyclopropylmethoxy)ethylphenoxy-3-(isopropylamino)-2-propanol hydrochloride
» Betaxolol Hydrochloride contains not less than 98.5 percent and not more than 101.5 percent of C18H29 NO3·HCl, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
It responds to the test for Chloride  191
191 when tested as directed for alkaloidal hydrochlorides.
when tested as directed for alkaloidal hydrochlorides.
Melting range, Class I  741
741 :
between 113
:
between 113 and 117
and 117 .
.
pH  791
791 :
between 4.5 and 6.5, in a solution (1 in 50).
:
between 4.5 and 6.5, in a solution (1 in 50).
Loss on drying  731
731 —
Dry it in vacuum at 65
—
Dry it in vacuum at 65 for 2 hours: it loses not more than 1.0% of its weight.
for 2 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Chromatographic purity—
Buffer—
Prepare a solution of 0.025 M monobasic potassium phosphate containing 0.1% (w/v) of tetrabutylammonium bromide. Adjust with 0.025 M phosphoric acid to a pH of 3.0.
Mobile phase—
Prepare a suitable filtered and degassed mixture of Buffer and acetonitrile (85:15). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Resolution solution—
Prepare a solution in Mobile phase containing 2.0 mg of USP Betaxolol Hydrochloride RS and 1 mg of alprenolol hydrochloride per mL.
Test preparation—
Prepare a solution of Betaxolol Hydrochloride in Mobile phase containing 2.0 mg per mL.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 273-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.9 for alprenolol and 1.0 for betaxolol; the resolution, R, between the two peaks is not less than 1.0; the tailing factors for the two peaks are not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
)—The liquid chromatograph is equipped with a 273-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.9 for alprenolol and 1.0 for betaxolol; the resolution, R, between the two peaks is not less than 1.0; the tailing factors for the two peaks are not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject about 20 µL of the Test preparation into the chromatograph, record the chromatogram, and measure the areas for the peaks. [note—Allow the elution to continue for about five times the elution time of the betaxolol peak before making the next injection. ] Calculate the percentage of each impurity by the same formula:
100(ri / rS)
in which ri is the response of each individual peak, other than the main betaxolol peak, in the chromatogram obtained from the Test preparation, and rS is the sum of the responses of all the peaks obtained in the chromatogram from the Test preparation: the sum of all impurities found is not more than 1.0%.
Assay—
Dissolve about 300 mg of Betaxolol Hydrochloride, accurately weighed, in 50 mL of glacial acetic acid. Add 7 mL of mercuric acetate TS, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 34.39 mg of C18H29NO3·HCl.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2348