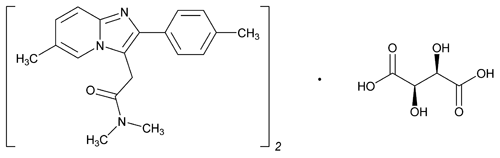
Zolpidem Tartrate
(C19H21N3O)2· C4H6O6 764.87
Imidazo[1,2-a]pyridine-3-acetamide, N,N,6-trimethyl-2-(4-methylphenyl)-, [R-(R*,R*)]-2,3-dihydroxybutanedioate;
N,N,6-Trimethyl-2-p-tolylimidazo[1,2-a]pyridine-3-acetamide l-(+)-tartrate

 [99294-93-6].
[99294-93-6].
(zol' pi dem tar' trate).
(C19H21N3O)2· C4H6O6 764.87
Imidazo[1,2-a]pyridine-3-acetamide, N,N,6-trimethyl-2-(4-methylphenyl)-, [R-(R*,R*)]-2,3-dihydroxybutanedioate;
N,N,6-Trimethyl-2-p-tolylimidazo[1,2-a]pyridine-3-acetamide l-(+)-tartrate
DEFINITION
Zolpidem Tartrate contains NLT 98.5% and NMT 101.0% of C42H48N6O8, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
Sample:
Dissolve 0.10 g in 10 mL of 0.1 M hydrochloric acid. Add 10 mL of water. Add dropwise with stirring 1 mL of dilute ammonia. Filter and collect the resulting precipitate. Wash the precipitate with water, and then dry at 100 –105
–105 for 2 h. Use 2 mg of the dried residue to make the KBr pellet.
for 2 h. Use 2 mg of the dried residue to make the KBr pellet.
Analysis:
The IR spectrum of the free base thus obtained corresponds to the IR spectrum of a similarly prepared KBr pellet with 2 mg of USP Zolpidem RS free base.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Organic Impurities.
• C. Reaction of Tartrates
Sample:
0.1 g of Zolpidem Tartrate
Analysis:
Dissolve the Sample in 1 mL of methanol with gentle heating. To 0.1 mL of the solution, add 0.1 mL of a 100-g/L solution of potassium bromide, 0.1 mL of a 20-g/L solution of resorcinol, and 3 mL of sulfuric acid. Heat on a water bath for 5–10 min. A dark blue color develops. Allow to cool, then pour the solution into water.
Acceptance criteria:
The dark blue color changes to red.
ASSAY
• Procedure
Sample solution:
Dissolve 0.300 g of Zolpidem Tartrate in a mixture of 20 mL of anhydrous acetic acid and 20 mL of acetic anhydride.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N perchloric acid VS
Endpoint detection:
Potentiometric
Analysis
Sample:
Sample solution
Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Carry out a blank titration.
Calculate the percentage of C42H48N6O8 in the portion of Zolpidem Tartrate taken:
Result = [(V  B) × N × F/W] × 100
B) × N × F/W] × 100
| V | = | = sample titrant volume (mL) |
| B | = | = blank titrant volume (mL) |
| N | = | = titrant normality (meq/mL) |
| F | = | = 382.4 mg/meq |
| W | = | = sample weight (mg) |
Acceptance criteria:
98.5%–101.0% on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure
Buffer:
5.6 g/L phosphoric acid in water. Adjust with triethylamine to a pH of 5.5.
Mobile phase:
Methanol, acetonitrile, and Buffer (23:18:59)
System suitability solution:
0.05 mg/mL each of USP Zolpidem Tartrate RS and USP Zolpidem Related Compound A RS in Mobile phase
Standard solution:
1 µg/mL of USP Zolpidem Tartrate RS in Mobile phase
Sample solution:
0.5 mg/mL of Zolpidem Tartrate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
3.9-mm × 15-cm; 4-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 2.0 between zolpidem tartrate related compound A and zolpidem tartrate, System suitability solution
Relative standard deviation:
NMT 10.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Zolpidem Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response for zolpidem from the Standard solution |
| CS | = | = concentration of USP Zolpidem Tartrate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Zolpidem Tartrate in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
NMT 0.10%
Total impurities:
NMT 0.2%
[Note—Disregard any peak with an area less than 0.25 times that of the principal peak from the Standard solution (0.05%) and any peak with a relative retention time of 0.16, which is due to tartaric acid. ]
SPECIFIC TESTS
• Water Determination, Method Ia  921
921 :
NMT 3.0%
:
NMT 3.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Zolpidem Related Compound A RS
N,N,7-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide.
C19H21N3O 307.39
N,N,7-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide.
C19H21N3O 307.39
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 5082
Pharmacopeial Forum: Volume No. 34(6) Page 1487