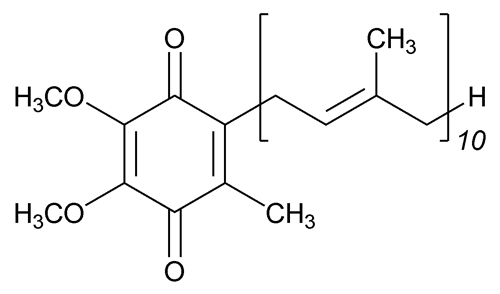
Ubidecarenone
C59H90O4 863.37
2,5-Cyclohexadiene-1,4-dione, 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl]-5,6-dimethoxy-3-methyl;
2-[(all-E)-3,7,11,15,19,23,27,31,35,39-Decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-p-benzoquinone

 [303-98-0].
[303-98-0].
C59H90O4 863.37
2,5-Cyclohexadiene-1,4-dione, 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl]-5,6-dimethoxy-3-methyl;
2-[(all-E)-3,7,11,15,19,23,27,31,35,39-Decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-p-benzoquinone
DEFINITION
Ubidecarenone (Coenzyme Q10) contains NLT 98.0% and NMT 101.0% of ubidecarenone (C59H90O4), calculated on the anhydrous basis.
IDENTIFICATION
• B.
Analysis:
Dissolve 50 mg of Ubidecarenone in 1 mL of ethyl ether, and add 10 mL of dehydrated alcohol. To 2 mL of this solution add 3 mL of dehydrated alcohol and 2 mL of dimethyl malonate. Add 1 mL of potassium hydroxide solution (1 in 5) dropwise.
Acceptance criteria:
A blue color appears.
ASSAY
• Procedure
Mobile phase:
Methanol and dehydrated alcohol (65:35)
System suitability solution:
0.5 mg/mL each of USP Ubidecarenone RS and USP Ubidecarenone Related Compound A RS in dehydrated alcohol. Heat at 50 for 2 min, if necessary, for complete dissolution.
for 2 min, if necessary, for complete dissolution.
Standard solution:
1.0 mg/mL of USP Ubidecarenone RS in dehydrated alcohol. Heat at 50 for 2 min, if necessary, for complete dissolution.
for 2 min, if necessary, for complete dissolution.
Sample solution:
1.0 mg/mL of Ubidecarenone in dehydrated alcohol. Heat at 50 for 2 min, if necessary, for complete dissolution.
for 2 min, if necessary, for complete dissolution.
Chromatographic system
Mode:
LC
Detector:
UV 275 nm
Column:
4.6-mm × 15-cm; packing L1
Column temperature:
35
Flow rate:
Adjust to obtain a retention time of about 11 min for ubidecarenone.
Injection size:
5 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for ubidecarenone related compound A and ubidecarenone are about 0.75 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 4 between ubidecarenone related compound A and ubidecarenone
Relative standard deviation:
NMT 0.8% for ubidecarenone
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of ubidecarenone (C59H90O4) in the portion of Ubidecarenone taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Ubidecarenone RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Ubidecarenone in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–101.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Chromatographic Purity
Procedure 1: Coenzymes Q7,Q8, Q9, Q11, and Related Impurities
Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay.
Analysis
Sample:
Sample solution
Calculate the percentage of impurities in the portion of Ubidecarenone taken:
Result = (rT1/rT2) × 100
| rT1 | = | = sum of all peak responses, other than that for ubidecarenone |
| rT2 | = | = sum of all peak responses |
Acceptance criteria:
NMT 1.0%
Procedure 2: Ubidecarenone (2Z)-Isomer and Related Impurities
Mobile phase:
n-Hexane and ethyl acetate (97:3)
System suitability solution:
1 mg/mL of USP Ubidecarenone for System Suitability RS in n-hexane
Sample solution:
1 mg/mL of Ubidecarenone in n-hexane
Chromatographic system
Mode:
LC
Detector:
UV 275 nm
Column:
4.6-mm × 25-cm; packing L3
Flow rate:
2 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for ubidecarenone (2Z)-isomer and ubidecarenone are about 0.85 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between the ubidecarenone (2Z)-isomer and ubidecarenone
Analysis
Sample:
Sample solution
Calculate the percentage of impurities in the portion of Ubidecarenone taken:
Result = (rT1/rT2) × 100
| rT1 | = | = sum of all peak responses, other than that for ubidecarenone |
| rT2 | = | = sum of all peak responses |
Acceptance criteria:
NMT 1.0%
Total impurities:
NMT 1.5%, obtained from Chromatographic Purity Procedures 1 and 2
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.2%
:
NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers.
• USP Reference Standards  11
11
USP Ubidecarenone for System Suitability RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1460
Pharmacopeial Forum: Volume No. 31(1) Page 86