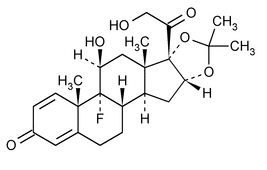
Triamcinolone Acetonide
(trye'' am sin' oh lone a seet' oh nide).
Pregna-1,4-diene-3,20-dione, 9-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (11
9-Fluoro-11
» Triamcinolone Acetonide contains not less than 97.0 percent and not more than 102.0 percent of C24H31FO6, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
A:
Infrared Absorption  197K
197K : recrystallized from methanol.
: recrystallized from methanol.
Loss on drying  731
731 —
Dry it in vacuum at 60
—
Dry it in vacuum at 60 for 4 hours: it loses not more than 1.5% of its weight.
for 4 hours: it loses not more than 1.5% of its weight.
Heavy metals—
Carefully ignite 1.0 g in a muffle furnace at about 550 until thoroughly charred. Cool, add to the contents of the crucible 5 drops of sulfuric acid and 2 mL of nitric acid, cautiously heat until reaction has ceased, then ignite in a muffle furnace at 500
until thoroughly charred. Cool, add to the contents of the crucible 5 drops of sulfuric acid and 2 mL of nitric acid, cautiously heat until reaction has ceased, then ignite in a muffle furnace at 500 to 600
to 600 until the carbon is entirely burned off. Cool, add 2 mL of hydrochloric acid, and slowly evaporate on a steam bath to dryness. Moisten the residue with 1 drop of hydrochloric acid and 5 mL of hot water, and digest for 2 minutes. Add 1 drop of phenolphthalein TS, then add 6 N ammonium hydroxide dropwise until the reaction is alkaline. Render the solution acid with 1 N acetic acid, then add 1 mL of excess, transfer to a beaker, and add water to make 10 mL. Pipet 2.5 mL (equivalent to 25 µg of lead) of Standard Lead Solution (see Lead
until the carbon is entirely burned off. Cool, add 2 mL of hydrochloric acid, and slowly evaporate on a steam bath to dryness. Moisten the residue with 1 drop of hydrochloric acid and 5 mL of hot water, and digest for 2 minutes. Add 1 drop of phenolphthalein TS, then add 6 N ammonium hydroxide dropwise until the reaction is alkaline. Render the solution acid with 1 N acetic acid, then add 1 mL of excess, transfer to a beaker, and add water to make 10 mL. Pipet 2.5 mL (equivalent to 25 µg of lead) of Standard Lead Solution (see Lead  231
231 ) into a second beaker, add 3 mL of water and 1 drop of phenolphthalein TS, render just alkaline with 6 N ammonium hydroxide, then render acid with 1 N acetic acid, and add 1 mL in excess. Dilute with water to 10 mL. To each beaker add 5 mL of freshly prepared hydrogen sulfide TS, mix, and allow to stand for 5 minutes. Pass each solution through a separate, acid-resistant, white, plain membrane filter of 0.22-µm pore size and 25 mm in diameter, collecting the precipitates on the filter disks: the color of the precipitate from the solution under test is not darker than that from the control. The heavy metals limit is 0.0025%.
) into a second beaker, add 3 mL of water and 1 drop of phenolphthalein TS, render just alkaline with 6 N ammonium hydroxide, then render acid with 1 N acetic acid, and add 1 mL in excess. Dilute with water to 10 mL. To each beaker add 5 mL of freshly prepared hydrogen sulfide TS, mix, and allow to stand for 5 minutes. Pass each solution through a separate, acid-resistant, white, plain membrane filter of 0.22-µm pore size and 25 mm in diameter, collecting the precipitates on the filter disks: the color of the precipitate from the solution under test is not darker than that from the control. The heavy metals limit is 0.0025%.
Chromatographic purity—
Mobile phase—
Prepare a filtered and degassed mixture of water and acetonitrile (17:8). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Test solution—
Transfer about 25 mg of Triamcinolone Acetonide, accurately weighed, to a 50-mL volumetric flask; dissolve in 25 mL of methanol, shake vigorously to aid dissolution; dilute with Mobile phase to volume; and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Test solution, and record the peak responses as directed for Procedure: the resolution, R, between triamcinolone acetonide and any impurity peak is not less than 1.0.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Test solution, and record the peak responses as directed for Procedure: the resolution, R, between triamcinolone acetonide and any impurity peak is not less than 1.0.
Procedure—
Inject about 20 µL of the Test solution into the chromatograph, record the chromatogram for not less than four times the retention time of triamcinolone acetonide, and measure all of the peak responses. Calculate the percentage of each impurity in the portion of Triamcinolone Acetonide taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity; and rs is the sum of the responses of all the peaks: not more than 0.3% of any individual impurity is found, and not more than 0.8% of total impurities is found.
Assay—
Mobile phase—
Prepare a solution of acetonitrile in water containing approximately 30% (v/v) of acetonitrile.
Internal standard solution—
Dissolve USP Fluoxymesterone RS in methanol to obtain a solution having a concentration of about 50 µg per mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Triamcinolone Acetonide RS in Internal standard solution to obtain a solution having a known concentration of about 75 µg per mL. Mix an accurately measured volume of the resulting solution with an equal volume of Mobile phase to obtain a Standard preparation containing about 37.5 µg of USP Triamcinolone Acetonide RS per mL.
Assay preparation—
Using about 37 mg of Triamcinolone Acetonide, accurately weighed, proceed as directed for Standard preparation.
Procedure—
Introduce equal volumes (between 15 µL and 25 µL) of the Assay preparation and the Standard preparation into a high-pressure liquid chromatograph (see Chromatography  621
621 ) operated at room temperature, by means of a suitable microsyringe or sampling valve. Adjust the operating parameters with Mobile phase on the column so that the separation of triamcinolone acetonide and internal standard is optimized, with a retention time of about 14.5 minutes for triamcinolone acetonide. Typically, the apparatus is fitted with a 4-mm × 30-cm column containing packing L1 and is equipped with a UV detector capable of monitoring absorbance at 254 nm, and a suitable recorder. In a suitable chromatogram, the coefficient of variation for five replicate injections of a single specimen is not more than 3.0%; and the resolution factor, R (see Chromatography
) operated at room temperature, by means of a suitable microsyringe or sampling valve. Adjust the operating parameters with Mobile phase on the column so that the separation of triamcinolone acetonide and internal standard is optimized, with a retention time of about 14.5 minutes for triamcinolone acetonide. Typically, the apparatus is fitted with a 4-mm × 30-cm column containing packing L1 and is equipped with a UV detector capable of monitoring absorbance at 254 nm, and a suitable recorder. In a suitable chromatogram, the coefficient of variation for five replicate injections of a single specimen is not more than 3.0%; and the resolution factor, R (see Chromatography  621
621 ), between the peaks for triamcinolone acetonide and fluoxymesterone is not less than 2.0. Measure the heights of the internal standard and triamcinolone acetonide peaks at the same retention times obtained from the Assay preparation and the Standard preparation. Calculate the quantity, in mg, of C24H31FO6 in the portion of Triamcinolone Acetonide taken by the formula:
), between the peaks for triamcinolone acetonide and fluoxymesterone is not less than 2.0. Measure the heights of the internal standard and triamcinolone acetonide peaks at the same retention times obtained from the Assay preparation and the Standard preparation. Calculate the quantity, in mg, of C24H31FO6 in the portion of Triamcinolone Acetonide taken by the formula:
1000C(RU / RS)
in which C is the concentration, in mg per mL, of USP Triamcinolone Acetonide RS in the Standard preparation; and RU and RS are the ratios of the peak heights of triamcinolone acetonide to the internal standard obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4923
Pharmacopeial Forum: Volume No. 31(3) Page 800