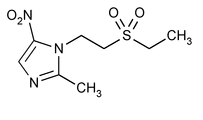
Tinidazole
(tye nye' da zole).
» Tinidazole contains not less than 98.0 percent and not more than 101.0 percent of C8H13N3O4S, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers, protected from light, at controlled room temperature.
Identification—
C:
The RF value and intensity of the principal spot obtained from the chromatogram of Test solution 2 correspond to those obtained from the chromatogram of Standard solution 1, as obtained in the test for Related compounds.
Melting range  741
741 :
between 125
:
between 125 and 128
and 128 .
.
Loss on drying  731
731 —
Dry it at a temperature between 100
—
Dry it at a temperature between 100 and 105
and 105 to constant weight: it loses not more than 0.5% of its weight.
to constant weight: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Related compounds—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution 1—
Dissolve about 200 mg of Tinidazole, accurately weighed, in 10 mL of methanol.
Test solution 2—
Transfer 1.0 mL of Test solution 1 to a 10-mL volumetric flask, dilute with methanol to volume, and mix.
Standard solution 1—
Prepare a solution of USP Tinidazole RS in methanol containing 2.0 mg per mL.
Standard solution 2—
Dilute 1.0 mL of Standard solution 1 with methanol to 20 mL.
Standard solution 3—
Dilute 4.0 mL of Standard solution 2 with methanol to 10 mL.
Standard solution 4—
Prepare a solution of USP Tinidazole Related Compound A RS in methanol containing 0.1 mg per mL.
Standard solution 5—
Prepare a solution of USP Tinidazole Related Compound B RS in methanol containing 0.1 mg per mL.
Application volume:
10 µL.
Developing solvent system:
a mixture of ethyl acetate and butyl alcohol (3:1).
Procedure—
Proceed as directed for Thin-Layer Chromatography under Chromatography  621
621 . Activate the plate for at least 1 hour at 110
. Activate the plate for at least 1 hour at 110 . Examine the plate under short-wavelength UV light: any spots due to tinidazole related compound A and tinidazole related compound B obtained from Test solution 1 are no more intense than the corresponding spots obtained from Standard solution 4 and Standard solution 5, respectively; any spot, other than the principal spot, obtained from Test solution 1 is not more intense than the spot obtained from Standard solution 2; and not more than one such spot is more intense than the spot obtained from Standard solution 3.
. Examine the plate under short-wavelength UV light: any spots due to tinidazole related compound A and tinidazole related compound B obtained from Test solution 1 are no more intense than the corresponding spots obtained from Standard solution 4 and Standard solution 5, respectively; any spot, other than the principal spot, obtained from Test solution 1 is not more intense than the spot obtained from Standard solution 2; and not more than one such spot is more intense than the spot obtained from Standard solution 3.
Assay—
Dissolve 150 mg of Tinidazole, accurately weighed, in 25.0 mL of glacial acetic acid, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically with suitable electrodes (see Titrimetry  541
541 ). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 24.73 mg of C8H13N3O4S.
). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 24.73 mg of C8H13N3O4S.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4874
Pharmacopeial Forum: Volume No. 27(1) Page 1820