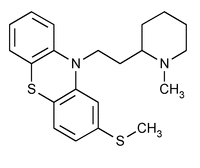
Thioridazine
C21H26N2S2 370.58
10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-;
10-[2-(1-Methyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine

 [50-52-2].
[50-52-2].
(thye'' oh rid' a zeen).
C21H26N2S2 370.58
10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-;
10-[2-(1-Methyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine
DEFINITION
Thioridazine contains NLT 99.0% and NMT 101.0% of C21H26N2S2, calculated on the dried basis.
[Note—Throughout the following procedures, protect samples, the Reference Standard, and the solutions containing them by conducting the procedures without delay, under subdued light, or using low-actinic glassware. ]
IDENTIFICATION
ASSAY
• Procedure
Sample solution:
300 mg of Thioridazine in 60 mL of glacial acetic acid
Analysis:
Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 37.06 mg of C21H26N2S2.
). Each mL of 0.1 N perchloric acid is equivalent to 37.06 mg of C21H26N2S2.
Acceptance criteria:
99.0%–101.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Organic Impurities
[Note—Conduct this procedure without delay, under subdued light. ]
Diluent:
Methanol and ammonium hydroxide (49:1)
Standard solution A:
50 µg/mL of USP Thioridazine RS in Diluent
Standard solution B:
20 µg/mL of USP Thioridazine RS in Diluent
Sample solution:
10 mg/mL of Thioridazine in Diluent
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
5 µL
Developing solvent system:
Chloroform, isopropyl alcohol, and ammonium hydroxide (74:25:1)
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Immediately develop until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, allow the solvent to evaporate, and examine the plate under short-wavelength UV light.
Acceptance criteria:
The chromatograms show principal spots at about the same RF value; no secondary spot, if present from the Sample solution, is more intense than the principal spot from Standard solution A (0.5%); and the sum of the intensities of all secondary spots, if present in the Sample solution, is NMT 0.5%.
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample in vacuum at 50
:
Dry a sample in vacuum at 50 for 4 h: it loses NMT 0.5% of its weight.
for 4 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4846