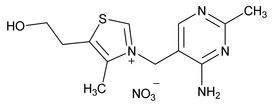Thiamine Mononitrate
C12H17N5O4S 327.36
Thiazolium, 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl-, nitrate (salt);
Thiamine nitrate (salt)

 [532-43-4].
[532-43-4].
(thye' a min mon'' oh nye' trate).
C12H17N5O4S 327.36
Thiazolium, 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl-, nitrate (salt);
Thiamine nitrate (salt)
DEFINITION
Thiamine Mononitrate contains NLT 98.0% and NMT 102.0% of thiamine mononitrate (C12H17N5O4S), calculated on the dried basis.
IDENTIFICATION
• A.
Sample solution:
20 mg/mL of Thiamine Mononitrate
Analysis:
To 2 mL of the Sample solution add 2 mL of sulfuric acid. Cool, and superimpose 2 mL of ferrous sulfate TS.
Acceptance criteria:
A brown ring is produced at the junction of the two liquids.
• B.
Sample:
5 mg
Analysis:
Dissolve the Sample in a mixture of 1 mL of lead acetate TS and 1 mL of 2.5 N sodium hydroxide. Heat for several min on a steam bath.
Acceptance criteria:
After dissolution of the Sample, a yellow color is produced. After heating the solution, the color changes to brown and on standing, a precipitate of lead sulfide separates.
• C.
A solution of Thiamine Mononitrate yields a white precipitate with mercuric chloride TS, and a red-brown precipitate with iodine TS. It also yields a precipitate with mercuric–potassium iodide TS, and with trinitrophenol TS.
• D.
Sample solution:
Dissolve 5 mg in 5 mL of 0.5 N sodium hydroxide.
Analysis:
To the Sample solution add 0.5 mL of potassium ferricyanide TS and 5 mL of isobutyl alcohol. Shake the mixture vigorously for 2 min, and allow the liquid layers to separate.
Acceptance criteria:
When illuminated from above by a vertical beam of UV light and viewed at a right angle to this beam, the air–liquid meniscus shows a vivid blue fluorescence, which disappears when the mixture is slightly acidified, but reappears when it is again made alkaline.
ASSAY
• Procedure
Solution A:
0.005 M sodium 1-octanesulfonate in dilute glacial acetic acid (1 in 100)
Solution B:
Methanol and acetonitrile (3:2)
Mobile phase:
Solution A and Solution B (60:40)
Internal standard solution:
2.0% (v/v) of methylbenzoate in methanol
Standard solution:
Prepare a 1-mg/mL solution of USP Thiamine Hydrochloride RS in Mobile phase. Transfer 20.0 mL of this solution and 5.0 mL of Internal standard solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume. The Standard solution contains 400 µg/mL of thiamine hydrochloride.
Sample solution:
Prepare a 2-mg/mL solution of Thiamine Mononitrate in Mobile phase. Transfer 10.0 mL of this solution and 5.0 mL of Internal standard solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4-mm × 30-cm; packing L1
Flow rate:
1 mL/min. [Note—The flow rate may be adjusted as needed to obtain a retention time of about 12 min for thiamine hydrochloride. ]
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 4.0 between the thiamine and methylbenzoate peaks
Tailing factor:
NMT 2.0 for the thiamine peak
Column efficiency:
NLT 1500 theoretical plates for the thiamine peak
Relative standard deviation:
NMT 2.0% for the ratios of the thiamine peak area to the internal standard peak area
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of thiamine mononitrate (C12H17N5O4S) in the portion of Thiamine Mononitrate taken:
Result = (RU/RS) × (CS/CU) × (Mr1/Mr2) × 100
| RU | = | = internal standard ratio (peak area of thiamine/peak area of the internal standard) from the Sample solution |
| RS | = | = internal standard ratio (peak area of thiamine/peak area of the internal standard) from the Standard solution |
| CS | = | = concentration of USP Thiamine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Thiamine Mononitrate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of thiamine mononitrate, 327.36 |
| Mr2 | = | = molecular weight of thiamine hydrochloride, 337.27 |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.2%
:
NMT 0.2%
• Chloride and Sulfate, Chloride  221
221
Standard:
0.40 mL of 0.020 N hydrochloric acid
Sample:
500 mg of Thiamine Mononitrate
Acceptance criteria:
NMT 0.06%
• Related Compounds
Solution A, Solution B, and Mobile phase:
Proceed as directed in the Assay.
Sample solution:
1.0 mg/mL of Thiamine Mononitrate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.0-mm × 15-cm; packing L1
Flow rate:
0.75 mL/min
Injection size:
10 µL
Analysis
Sample:
Sample solution
Allow the Sample solution to elute for NLT three times the retention time of the main peak.
Calculate the percentage of total secondary peaks in the portion of Thiamine Mononitrate taken:
Result = (rU/rT) × 100
| rU | = | = sum of the areas of all the peaks, except that of the thiamine peak |
| rT | = | = sum of the areas of all the peaks |
Acceptance criteria:
NMT 1.0%
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry 500 mg at 105
:
Dry 500 mg at 105 for 2 h: it loses NMT 1.0% of its weight.
for 2 h: it loses NMT 1.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4837