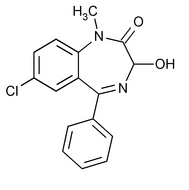
Temazepam
C16H13ClN2O2 300.74
2H-1,4-Benzodiazepin-2-one, 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-;
7-Chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one

 [846-50-4].
[846-50-4].
 Buffer:
2.7 g/L of monobasic potassium phosphate. Adjust with phosphoric acid to a pH of 3.0.
Buffer:
2.7 g/L of monobasic potassium phosphate. Adjust with phosphoric acid to a pH of 3.0.
(tem az' e pam).
C16H13ClN2O2 300.74
2H-1,4-Benzodiazepin-2-one, 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-;
7-Chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one
DEFINITION
Temazepam contains NLT 98.0% and NMT 102.0% of C16H13ClN2O2, calculated on the dried basis. [Caution—Temazepam is a potent sedative: its powder should not be inhaled.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
Change to read:
• Procedure
Mobile phase:
Acetonitrile and Buffer (47:53)
Diluent:
Methanol and water (90:10)
Standard solution:
0.2 mg/mL of USP Temazepam RS in Diluent
Sample solution:
0.2 mg/mL of Temazepam in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4-mm × 25-cm; 5-µm packing L16
Flow rate:
2 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 800 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of temazepam (C16H13ClN2O2) in the portion of sample taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Temazepam RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Temazepam in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis USP35
USP35
IMPURITIES
Organic Impurities
• Procedure
Standard stock solution:
10 mg/mL of USP Temazepam RS in chloroform
Standard solution A:
1 mg/mL from the Standard stock solution in chloroform
Standard solution B:
0.05 mg/mL from the Standard stock solution in chloroform
Sample solution:
10 mg/mL of Temazepam in chloroform
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
10 µL
Developing solvent system:
Cyclohexane, chloroform, methanol, and ammonium hydroxide (50:40:12:1)
Analysis:
Develop the chromatograms in the Developing solvent system until the solvent front has moved about 10 cm above the point of application. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Sample solution with those of the principal spots in the chromatograms of the Standard solutions.
Acceptance criteria:
No secondary spot from the Sample solution is greater in intensity than 1.0%. The sum of the intensities of secondary spots from the Sample solution corresponds to NMT 2.0%.
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 2 h: it loses NMT 0.5% of its weight.
for 2 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4780
Pharmacopeial Forum: Volume No. 36(6) Page 1574