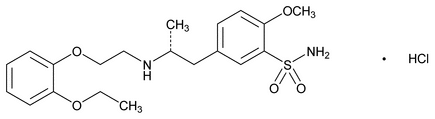
Tamsulosin Hydrochloride
C20H28N2O5S · HCI 444.97
Benzenesulfonamide, 5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxy-, monohydrochloride, (R)-;
( )-(R)-5-[2-[[2-(o-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide monohydrochloride
)-(R)-5-[2-[[2-(o-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide monohydrochloride


 [106463-17-6].
[106463-17-6].
(tam soo' loe sin hye'' droe klor' ide).
C20H28N2O5S · HCI 444.97
Benzenesulfonamide, 5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxy-, monohydrochloride, (R)-;
(
DEFINITION
Tamsulosin Hydrochloride contains NLT 98.5% and NMT 101.0% of (C20H28N2O5S·HCl), calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191
Sample solution:
7.5 mg/mL in water. [Note—Heat to dissolve. ] In an ice bath, cool 5 mL of the solution. Add 3 mL of diluted nitric acid, and shake until mixed thoroughly. Allow to stand for 30 min at room temperature, and filter.
ASSAY
• Procedure
Sample:
350 mg, previously dried at 105 for 2 h
for 2 h
Analysis:
Dissolve the Sample in 5 mL of formic acid, and add 75 mL of a mixture of glacial acetic acid and acetic anhydride (3:2). Titrate immediately with 0.1 N perchloric acid VS and determine the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 44.50 mg of C20H28N2O5S·HCl.
). Each mL of 0.1 N perchloric acid is equivalent to 44.50 mg of C20H28N2O5S·HCl.
IMPURITIES
Organic Impurities
• Procedure 1
[Note—For impurities eluting before tamsulosin. ]
Buffer:
Dissolve 8.7 mL of perchloric acid (70%) and 3.0 g of sodium hydroxide in 1900 mL of water. Adjust with 1 N sodium hydroxide to a pH of 2.0, and add sufficient water to make 2000 mL.
Mobile phase:
Acetonitrile and Buffer (3:7)
System suitability solution:
25 µg /mL of Tamsulosin Hydrochloride and 50 µg/mL of propylparaben in Mobile phase
Sample solution:
5.0 mg/mL of Tamsulosin Hydrochloride in Mobile phase
Standard solution:
10 µg/mL of Tamsulosin Hydrochloride, from the Sample solution in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm x 15-cm; 5-µm packing L1
Temperature:
40
Flow rate:
1.3 mL/min
Injection size:
10 µL
[Note—Record the chromatogram for NLT 1.5 times the retention time of tamsulosin. ]
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 12 between tamsulosin and propylparaben, System suitability solution. [Note—The elution order is tamsulosin followed by propylparaben. ]
Relative standard deviation:
NMT 4% for six replicate injections, Standard solution
Analysis
Samples:
Sample solution and Standard solution
Calculate the percentage of any individual impurity, eluting before the tamsulosin peak, in the portion of Tamsulosin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity eluting before tamsulosin from the Sample solution |
| rS | = | = peak response of tamsulosin from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
NMT 0.10% for any individual impurity. [Note—If present, des-ethoxy impurity and methoxy impurity eluting at the relative retention time of about 0.8 are not separated by this method and should be integrated together to determine conformance. (Des-ethoxy impurity is 2-methoxy-5-[(2R)-2-[(2-phenoxyethyl)amino]propyl]benzenesulfonamide, and methoxy impurity is 2-methoxy-5-[(2R)-2-[[2-(2-methoxyphenoxy)ethyl]amino]propyl]benzenesulfonamide.) NMT 0.15% of the sum of des-ethoxy and methoxy impurities is found. ]
[Note—Reporting level for impurities is 0.05%. ]
• Procedure 2
[Note—For impurities eluting after tamsulosin. ]
Buffer, Standard solution, and Sample solution:
Proceed as directed for Procedure 1.
[Note—Use the Mobile phase in Procedure 1 to prepare the Standard solution and Sample solution. ]
Mobile phase:
Acetonitrile and Buffer (1:1)
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm x 15-cm; 5-µm packing L1
Temperature:
40
Flow rate:
1.0 mL/min
Injection size:
10 µL
[Note—Record the chromatogram for NLT 5 times the retention time of tamsulosin. ]
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
Use a column that meets the resolution requirements of Procedure 1.
Relative standard deviation:
NMT 4% for six replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity, eluting after the tamsulosin peak, in the portion of Tamsulosin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity eluting after tamsulosin from the Sample solution |
| rS | = | = peak response of tamsulosin from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
NMT 0.10% for any individual impurity. [Note—Reporting level for impurities is 0.05%. ]
• Total impurities:
NMT 0.2%, including all impurities in Procedure 1 and Procedure 2
SPECIFIC TESTS
• Enantiomeric Purity
Mobile phase:
Hexane, dehydrated alcohol, methanol, and diethylamine (650:200:150:1)
System suitability solution:
40 µg/mL of USP Racemic Tamsulosin Hydrochloride RS in methanol
Sample solution:
2.0 mg/mL of Tamsulosin Hydrochloride in methanol
Standard solution:
2 µg/mL of Tamsulosin Hydrochloride, from the Sample solution, in methanol
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; packing L51
Temperature:
40
Flow rate:
0.5 mL/min
Injection size:
10 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for the optical isomer and tamsulosin are 0.8 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 2 between the optical isomer and tamsulosin
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the optical isomer in the portion of Tamsulosin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of the optical isomer from the Sample solution |
| rS | = | = peak response of tamsulosin from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
NMT 0.3% of the optical isomer
• Optical Rotation, Specific Rotation  781S
781S :
:
 17.5
17.5 to
to  20.5
20.5 at 20
at 20
Sample solution:
7.5 mg/mL in water. [Note—Sample should be previously dried at 105 for 2 h; heat at 60
for 2 h; heat at 60 –70
–70 to dissolve, and allow to cool before using. ]
to dissolve, and allow to cool before using. ]
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 2 h: it loses NMT 0.5% of its weight.
for 2 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Racemic Tamsulosin Hydrochloride RS
USP Tamsulosin Hydrochloride RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4745
Pharmacopeial Forum: Volume No. 35(3) Page 578