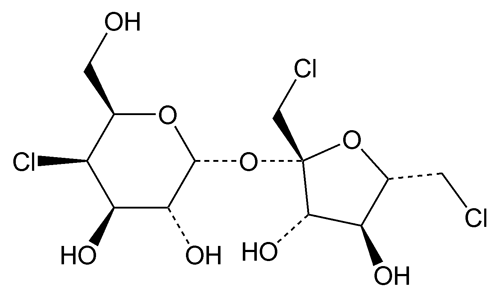
Sucralose
C12H19Cl3O8 397.64
1,6-Dichloro-1,6-dideoxy- -d-fructofuranosyl-4-chloro-4-deoxy-
-d-fructofuranosyl-4-chloro-4-deoxy- -d-galactopyranoside;
-d-galactopyranoside;
1¢,4,6¢-Trichlorogalactosucrose

 [56038-13-2].
[56038-13-2].
(soo' kra lose).
C12H19Cl3O8 397.64
1,6-Dichloro-1,6-dideoxy-
1¢,4,6¢-Trichlorogalactosucrose
DEFINITION
Sucralose contains NLT 98.0% and NMT 102.0% of C12H19Cl3O8, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the principal peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C.
The RF value of the principal spot of the Sample solution corresponds to that of Standard solution A, as obtained in the test for Related Compounds.
ASSAY
• Procedure
Mobile phase:
Acetonitrile and water (3:17)
Standard solution:
1 mg/mL of USP Sucralose RS in Mobile phase
Sample solution:
1 mg/mL of Sucralose in Mobile phase
Chromatographic system
Mode:
LC
Detector:
Refractive index
Column:
8-mm × 10-cm; packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The retention time of sucralose is about 9 min. ]
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of sucralose (C12H19Cl3O8) in the portion of Sucralose taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of the Sample solution |
| rS | = | = peak response of the Standard solution |
| CS | = | = concentration of USP Sucralose RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Sucralose in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.7%
:
NMT 0.7%
• Heavy Metals, Method II  231
231 :
10 ppm
:
10 ppm
• Limit of Methanol
Internal standard solution:
0.1 µL/mL of n-propyl alcohol in pyridine
Standard solution:
0.2 µL/mL of methanol in Internal standard solution
Sample solution:
0.2 g/mL of Sucralose in Internal standard solution
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
4-mm × 2-m glass column; packed with 80- to 100-mesh silanized support S6
Temperature
Column:
150
Detector:
250
Injector:
200
Carrier gas:
Helium
Flow rate:
20 mL/min
Injection size:
1 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of methanol in the portion of Sucralose taken:
Result = (RU/RS) × [(CS/CU) × F1] × F2 × 100
| RU | = | = peak response ratio of methanol to n-propyl alcohol, from the Sample solution |
| RS | = | = peak response ratio of methanol to n-propyl alcohol, from the Standard solution |
| CS | = | = concentration of methanol in the Standard solution (µL/mL) |
| CU | = | = concentration of Sucralose in the Sample solution (g/mL) |
| F1 | = | = conversion factor from µL to mL |
| F2 | = | = specific gravity of methanol, 0.79 g/cm3 |
Acceptance criteria:
NMT 0.1%
• Related Compounds
Adsorbent:
0.20-mm layer of octadecylsilanized chromatographic silica gel. The thin-layer chromatographic plate also has a preadsorbent zone.
Detection reagent:
Sulfuric acid in methanol (3 in 20)
Standard solution A:
10.0 mg/mL of USP Sucralose RS in methanol
Standard solution B:
0.5 mL Standard solution A diluted to 10.0 mL with methanol
Sample solution:
100.0 mg/mL of Sucralose in methanol
Developing solvent system:
Acetonitrile and sodium chloride solution (1 in 20) (3:7)
Application volume:
5 µL
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Proceed as directed under Chromatography  621
621 , Thin-Layer Chromatography. Spray the plate with Detection reagent. Heat the plate for 10 min at 125
, Thin-Layer Chromatography. Spray the plate with Detection reagent. Heat the plate for 10 min at 125 .
.
Acceptance criteria:
The RF value of the principal spot from the Sample solution corresponds to that obtained from Standard solution A, and the color of any other single spot from the Sample solution is not more intense than that of the principal spot from Standard solution B (0.5%).
• Limit of Hydrolysis Products
[Note—This test does not require a developing solvent. ]
Adsorbent:
0.25-mm layer of chromatographic silica gel
Spray reagent:
12.3 mg/mL of p-anisidine and 16.6 mg/mL of phthalic acid in methanol. Store the solution in the dark and refrigerate to prevent discoloration. Discard if the solution becomes discolored. [Caution—p-Anisidine is toxic if inhaled or if absorbed through the skin.
]
Standard solution A:
100 mg/mL of mannitol
Standard solution B:
0.4 mg/mL of fructose and 100 mg/mL of mannitol
Sample solution:
250 mg/mL of Sucralose in methanol
Application volume:
5-µL portions separately applied in 1-µL increments, allowing the plate to dry between applications
Analysis
Samples:
Standard solution and Sample solution
Proceed as directed under Chromatography  621
621 , Thin-Layer Chromatography. Spray the plate with Spray reagent, and heat the plate at 100 ± 2
, Thin-Layer Chromatography. Spray the plate with Spray reagent, and heat the plate at 100 ± 2 for 15 min. If the spot from Standard solution A has darkened, repeat the test, heating for a shorter period of time. Immediately after heating, view the plate against a dark background.
for 15 min. If the spot from Standard solution A has darkened, repeat the test, heating for a shorter period of time. Immediately after heating, view the plate against a dark background.
Acceptance criteria:
The color of the spot from the Sample solution is not more intense than that from Standard solution B (0.1%).
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
+84.0
:
+84.0 to +87.5
to +87.5 at 20
at 20
Sample solution:
10 mg/mL of Sucralose
• Water Determination, Method I  921
921 :
NMT 2.0%
:
NMT 2.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, in a cool, dry place, at a temperature not exceeding 21 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1993
Pharmacopeial Forum: Volume No. 33(6) Page 1255