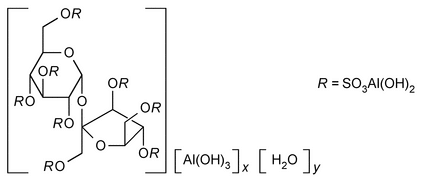Sucralfate
(soo kral' fate).
» Sucralfate is the hydrous basic aluminum salt of sucrose octasulfate. It contains the equivalent of not less than 30.0 percent and not more than 38.0 percent of sucrose octasulfate (C12H14O35S8).
Packaging and storage—
Preserve in tight containers.
USP Reference standards  11
11 —
—
USP Potassium Sucrose Octasulfate RS
[Note—Sucrosofate Potassium is USAN].
 -d-Glucopyranoside, 1,3,4,6-tetra-O-sulfo-
-d-Glucopyranoside, 1,3,4,6-tetra-O-sulfo- -d-fructofuranosyl, tetrakis (hydrogen sulfate), octapotassium salt, heptahydrate.
-d-fructofuranosyl, tetrakis (hydrogen sulfate), octapotassium salt, heptahydrate.
C12H14K8O35S8·7H2O 1413.64

 [CAS-76578-81-9].
[CAS-76578-81-9].
Anhydrous.
C12H14K8O35S8 1287.53

 [CAS-73264-44-5].
[CAS-73264-44-5].
[Note—Sucrosofate Potassium is USAN].
C12H14K8O35S8·7H2O 1413.64
Anhydrous.
C12H14K8O35S8 1287.53
Clarity and color of solution—
Dissolve 1.0 g in 10 mL of 2 N sulfuric acid: the solution is clear and practically colorless.
Identification—
A:
The retention time of the sucrose octasulfate peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
B:
Add 100 mL of 0.1 N hydrochloric acid to about 500 mg of Sucralfate, and boil the mixture gently, with stirring, for 20 minutes until the sample is completely dissolved. Neutralize with 0.1 N sodium hydroxide, and allow to cool. Add 4 mL of alkaline cupric tartrate TS. Boil a small amount of this solution: a red precipitate of cuprous oxide is produced.
C:
A solution in 3 N hydrochloric acid meets the requirements of the tests for Aluminum  191
191 .
.
Acid-neutralizing capacity—
Transfer about 250 mg, accurately weighed, to a 250-mL screw-capped bottle, add 100.0 mL of 0.1 N hydrochloric acid, previously heated to 37 , cap the bottle, place it in a 37
, cap the bottle, place it in a 37 water bath, and stir the contents continuously for 1 hour. Cool to room temperature, and transfer 20.0 mL to a 100-mL beaker. Add 30 mL of water, and titrate with 0.1 N sodium hydroxide VS to a pH of 3.5. Perform a blank titration on a mixture of water and 0.1 N hydrochloric acid (30:20.0). Calculate the mEq of acid consumed per g of Sucralfate taken by the formula:
water bath, and stir the contents continuously for 1 hour. Cool to room temperature, and transfer 20.0 mL to a 100-mL beaker. Add 30 mL of water, and titrate with 0.1 N sodium hydroxide VS to a pH of 3.5. Perform a blank titration on a mixture of water and 0.1 N hydrochloric acid (30:20.0). Calculate the mEq of acid consumed per g of Sucralfate taken by the formula:
5N(VB – VT)/W
where N is the exact normality of the sodium hydroxide VS; VB and VT are the volumes, in mL, of sodium hydroxide VS consumed by the blank and the test solution, respectively; and W is the weight, in g, of Sucralfate taken: not less than 12 mEq of acid is consumed.
Chloride  221
221 —
Transfer 500 mg to a 100-mL volumetric flask, add 30 mL of 2 N nitric acid, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL color comparison tube, add 3 mL of 2 N nitric acid and 2 mL of silver nitrate TS, dilute with water to 50 mL, and mix. Allow to stand, protected from direct sunlight, for 5 minutes. The sample shows no more turbidity than that produced in a solution containing 0.35 mL of 0.020 N hydrochloric acid: not more than 0.50% of chloride is found.
—
Transfer 500 mg to a 100-mL volumetric flask, add 30 mL of 2 N nitric acid, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL color comparison tube, add 3 mL of 2 N nitric acid and 2 mL of silver nitrate TS, dilute with water to 50 mL, and mix. Allow to stand, protected from direct sunlight, for 5 minutes. The sample shows no more turbidity than that produced in a solution containing 0.35 mL of 0.020 N hydrochloric acid: not more than 0.50% of chloride is found.
Arsenic, Method II  211
211 :
4 ppm.
:
4 ppm.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Limit of pyridine and 2-methylpyridine—
Internal standard solution—
Transfer 1.0 mL of 3-methylpyridine to a 50-mL volumetric flask, dilute with chloroform to volume, and mix. Transfer 1.0 mL of this solution to a 50-mL volumetric flask, dilute with chloroform to volume, and mix.
Standard stock solution—
Transfer about 0.5 g each of 2-methylpyridine and pyridine to a 50-mL volumetric flask, dissolve in chloroform, dilute with chloroform to volume, and mix. Quantitatively dilute 5.0 mL of this solution with chloroform to 50.0 mL. Transfer 5.0 mL of this solution to a 50.0 mL volumetric flask, dilute with chloroform to volume, and mix.
Standard solution—
Transfer 5.0 mL of Standard stock solution to a 20-mL volumetric flask, add 1.0 mL of the Internal standard solution, dilute with chloroform to volume, and mix.
Test solution—
Sonicate about 1 g of Sucralfate, accurately weighed, in 10.0 mL of 1 M sodium hydroxide until a uniformly cloudy mixture is obtained. Extract this solution with three 5-mL portions of chloroform, and collect the chloroform extracts in a 20-mL volumetric flask. Add 1.0 mL of the Internal standard solution, dilute with chloroform to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector, a split injection system, and a 0.53-mm × 10-m capillary column coated with a 2.65-µm layer of phase G27. Helium is used as the carrier gas, at a pressure of 36-mm of mercury. The column temperature is maintained at 50
)—
The gas chromatograph is equipped with a flame-ionization detector, a split injection system, and a 0.53-mm × 10-m capillary column coated with a 2.65-µm layer of phase G27. Helium is used as the carrier gas, at a pressure of 36-mm of mercury. The column temperature is maintained at 50 . The injection port temperature and the detector temperature are maintained at 150
. The injection port temperature and the detector temperature are maintained at 150 and 200
and 200 , respectively. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times of pyridine, 2-methylpyridine, and 3-methylpyridine are about 0.42, 0.72, and 1.0, respectively; the resolution, R, between pyridine and 2-methylpyridine is not less than 3.5; the resolution, R, between 2-methylpyridine and 3-methylpyridine is not less than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
, respectively. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times of pyridine, 2-methylpyridine, and 3-methylpyridine are about 0.42, 0.72, and 1.0, respectively; the resolution, R, between pyridine and 2-methylpyridine is not less than 3.5; the resolution, R, between 2-methylpyridine and 3-methylpyridine is not less than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Separately calculate the quantities, in µg, of pyridine and 2-methylpyridine, if present, in the portion of Sucralfate taken by the formula:
20C(RU / RS)
in which C is the concentration, in µg per mL, of pyridine or 2-methylpyridine in the Standard solution; and RU and RS are the peak response ratios of the analyte to the internal standard obtained from the Test solution and the Standard solution, respectively: not more than 0.05% each of pyridine and 2-methylpyridine is found.
Limit of sucrose heptasulfate—
Mobile phase—
Dissolve 99.1 g of ammonium sulfate in 900 mL of water, dilute with water to 1000 mL, and mix. Adjust with phosphoric acid to a pH of 3.5 ± 0.1, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Prepare as directed for the Standard preparation in the Assay.
Chromatographic system—
Prepare as directed in the Assay.
Test solution—
Prepare as directed for Assay preparation in the Assay.
Procedure—
Inject about 50 µL of the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The relative retention times are about 0.6 for sucrose heptasulfate and 1.0 for sucrose octasulfate. The ratio of the peak response of the sucrose heptasulfate peak to that of the sucrose octasulfate peak is not more than 0.1.
Aluminum content—
Transfer about 1.0 g, accurately weighed, to a 250-mL volumetric flask, add 10 mL of 6.0 N hydrochloric acid, mix, and heat with continuous stirring in a water bath at 70 for 5 minutes. Cool to room temperature, dilute with water to volume, and mix. Filter the solution, discarding the first portion of the filtrate. Transfer 25.0 mL of the filtrate to a 250-mL beaker, add 25.0 mL of 0.05 M edetate disodium VS, add 20 mL of acetic acid-ammonium acetate buffer TS, and mix. Heat in a water bath at 70
for 5 minutes. Cool to room temperature, dilute with water to volume, and mix. Filter the solution, discarding the first portion of the filtrate. Transfer 25.0 mL of the filtrate to a 250-mL beaker, add 25.0 mL of 0.05 M edetate disodium VS, add 20 mL of acetic acid-ammonium acetate buffer TS, and mix. Heat in a water bath at 70 for 5 minutes. Cool to room temperature, add 50 mL of alcohol and 2 mL of dithizone TS, and mix. Titrate with 0.05 M zinc sulfate VS to a bright rose-pink color. Perform a blank determination and make any necessary correction. Each mL of 0.05 M edetate disodium consumed is equivalent to 1.349 mg of aluminum: between 15.5% and 18.5% of aluminum is found, calculated on an “as is” basis.
for 5 minutes. Cool to room temperature, add 50 mL of alcohol and 2 mL of dithizone TS, and mix. Titrate with 0.05 M zinc sulfate VS to a bright rose-pink color. Perform a blank determination and make any necessary correction. Each mL of 0.05 M edetate disodium consumed is equivalent to 1.349 mg of aluminum: between 15.5% and 18.5% of aluminum is found, calculated on an “as is” basis.
Assay—
Mobile phase—
Dissolve 132 g of ammonium sulfate in 900 mL of water, dilute with water to 1000 mL, and mix. Adjust with phosphoric acid to a pH of 3.5 ± 0.1, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Potassium Sucrose Octasulfate RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 10 mg of anhydrous potassium sucrose octasulfate (as determined from the concentration of USP Potassium Sucrose Octasulfate RS corrected for water content by a titrimetric water determination) per mL.
Assay preparation—
Transfer about 450 mg of Sucralfate, accurately weighed, to a 35-mL centrifuge tube, and shake at a moderate rate on a vortex mixer. While shaking add 10.0 mL of a mixture of 4.0 N sulfuric acid and 2.2 N sodium hydroxide (1:1). Sonicate with swirling for 5 minutes, keeping the temperature of the mixture below 30 . Without delay transfer the tube to a vortex mixer and while shaking at moderate rate, add an accurately measured volume, V, in mL, of 0.1 N sodium hydroxide to bring the pH of the solution to approximately 2, and dilute the solution with (15.0
. Without delay transfer the tube to a vortex mixer and while shaking at moderate rate, add an accurately measured volume, V, in mL, of 0.1 N sodium hydroxide to bring the pH of the solution to approximately 2, and dilute the solution with (15.0  V) mL of water. Shake for 1 minute, and centrifuge for 5 minutes. Separate the clear supernatant layer, and allow it to stand at room temperature until the pH stabilizes. If the pH is not between 2.3 and 3.5, repeat the test using a different volume of 0.1 N sodium hydroxide. Use the clear supernatant layer.
V) mL of water. Shake for 1 minute, and centrifuge for 5 minutes. Separate the clear supernatant layer, and allow it to stand at room temperature until the pH stabilizes. If the pH is not between 2.3 and 3.5, repeat the test using a different volume of 0.1 N sodium hydroxide. Use the clear supernatant layer.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and a 3.9-mm × 30-cm column that contains packing L8. The detector and column temperatures are maintained at 30
)—
The liquid chromatograph is equipped with a refractive index detector and a 3.9-mm × 30-cm column that contains packing L8. The detector and column temperatures are maintained at 30 . The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency determined from the sucrose octasulfate peak is not less than 400 theoretical plates; the tailing factor for the sucrose octasulfate peak is not more than 4.0; and the relative standard deviation for replicate injections is not more than 2.0%.
. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency determined from the sucrose octasulfate peak is not less than 400 theoretical plates; the tailing factor for the sucrose octasulfate peak is not more than 4.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of sucrose octasulfate (C12H14O35S8) in the portion of Sucralfate taken by the formula:
(974.75/1287.53)(25C)(rU / rS)
in which 974.75 and 1287.53 are the molecular weights of sucrose octasulfate and anhydrous potassium sucrose octasulfate, respectively; C is the concentration, in mg per mL, of anhydrous potassium sucrose octasulfate in the Standard preparation; and rU and rS are the peak responses of sucrose octasulfate obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4695
Pharmacopeial Forum: Volume No. 33(2) Page 254