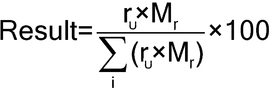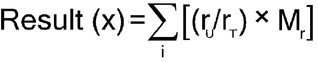Benzalkonium Chloride Solution
DEFINITION
Benzalkonium Chloride Solution contains NLT 95.0% and NMT 105.0% of the labeled amount of benzalkonium chloride in a solution that has a concentration of 1.0% or more; and NLT 93.0% and NMT 107.0% of the labeled amount in a solution that has a concentration of less than 1.0%. It may contain a suitable coloring agent and may contain NMT 10% of alcohol.
[Caution–Mixing Benzalkonium Chloride Solution with ordinary soaps and with anionic detergents may decrease or destroy the bacteriostatic activity of the Solution.
]
IDENTIFICATION
• A. Procedure
Analysis:
Add 1 mL of 2 N nitric acid to 2 mL of a solution having an equivalent to 10 mg/mL of benzalkonium chloride.
Acceptance criteria:
A white precipitate is formed, and it is dissolved after adding 5 mL of alcohol.
• B. Identification Tests—General, Chloride  191
191 :
A solution of it in a mixture of equal volumes of water and alcohol meets the requirements.
:
A solution of it in a mixture of equal volumes of water and alcohol meets the requirements.
• C. Procedure
Analysis:
Dissolve the residue obtained by evaporating on a steam bath, a volume of Solution equivalent to 200 mg of benzalkonium chloride in 1 mL of sulfuric acid, add 100 mg of sodium nitrate, and heat on a steam bath for 5 min. Cool, dilute with water to 10 mL, add 500 mg of zinc dust, and warm for 5 min on a steam bath. To 2 mL of the clear supernatant add 1 mL of sodium nitrite solution (1 in 20), cool in ice water, then add 3 mL of a solution of 500 mg of 2-naphthol in 10 mL of 6 N ammonium hydroxide.
Acceptance criteria:
An orange-red color is produced.
• D.
The retention times of the major peaks for benzalkonium chloride in the Sample solution correspond to those of the Standard solution, as obtained in the test for Ratio of Alkyl Components.
ASSAY
• Ratio of Alkyl Components
Solution A:
Adjust a 0.1 M solution of sodium acetate with glacial acetic acid to a pH of 5.0.
Mobile phase:
Acetonitrile and Solution A (9:11). Acetonitrile and Solution A may be adjusted from (2:3) to (3:2) to meet system suitability requirements.
Standard solution:
4 mg/mL of benzalkonium chloride, prepared from USP Benzalkonium Chloride RS and water
Sample solution:
Transfer a volume of Solution, equivalent to 400 mg of benzalkonium chloride, to a 100-mL volumetric flask, and dilute with water to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
3.9-mm × 30-cm; packing L10, or 4.6-mm × 25-cm; 10-µm packing L10
Flow rate:
2 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention time table below. Relative retention times are provided for information only, and the standard should be used to ensure appropriate peak identification. ]
| Name | Relative Retention Time |
|---|---|
| C10 homolog | 0.9 |
| C12 homolog | 1.0 |
| C14 homolog | 1.3 |
| C16 homolog | 1.7 |
Suitability requirements
Resolution:
NLT 1.5 between the C12 and C14 peaks
Relative standard deviation:
NMT 2.0% for the C12 peak
Analysis
Samples:
Standard solution and Sample solution
Identify the homolog peaks by comparison of the retention times with those from the Standard solution.
Calculate the percentage of each quaternary ammonium homolog taken:
| rU | = | = area of the peak due to a given homolog in the chromatogram from the Sample solution |
| Mr | = | = molecular weight of a given homolog. The molecular weights of the C10, C12, C14, and C16 homologs are 312, 340, 368, and 396, respectively. |
Acceptance criteria:
On the anhydrous basis, the content of the n-C12H25 homolog is NLT 40.0%, and the content of the n-C14H29 homolog is NLT 20.0% of the total alkylbenzyldimethylammonium chloride content. The amount of the n-C12H25 and n-C14H29 homolog components together is NLT 70.0% of the total alkylbenzyldimethylammonium chloride content.
• Total Alkylbenzyldimethylammonium Chlorides
Sample solution:
Evaporate or dilute with water to 30 mL a volume of Solution, equivalent to 500 mg of benzalkonium chloride.
Analysis:
Transfer the Sample solution with the aid of a minimum quantity of water to a glass-stoppered, 250-mL conical separator. Transfer 25 mL of methylene chloride. Add 10 mL of 0.1 N sodium hydroxide, and 10.0 mL of freshly prepared potassium iodide solution (1 in 20), insert the stopper in the separator, shake, allow the layers to separate, and discard the methylene chloride layer. Wash the aqueous layer with three 10-mL portions of methylene chloride, and discard the washings. Transfer the aqueous layer to a glass-stoppered, 250-mL conical flask, and rinse the separator with three 5-mL portions of water, adding the washings to the flask. Add 40 mL of cold hydrochloric acid to the flask, mix, and titrate with 0.05 M potassium iodate VS until the solution becomes light brown in color. Add 5 mL of methylene chloride, insert the stopper into the flask, and shake vigorously. Continue the titration, dropwise, with shaking after each addition, until the methylene chloride layer becomes colorless and the aqueous layer is clear yellow. Record the titrant volume, Vt (mL). Perform a blank determination, using 20 mL of water as the sample, and record the titrant volume, Vb (mL). [Note—Vb > Vt. ] The difference between the two titrations represents the amount of potassium iodate equivalent to the weight of benzalkonium chloride in the sample. Each mL of 0.05 M potassium iodate is equivalent to x/10 mg of benzalkonium chloride, where x represents the average molecular weight of the sample, derived by summing, for all homologs, the products:
| rU | = | = area of the peak produced by a given homolog in the chromatogram from the Ratio of Alkyl Components test |
| rT | = | = sum of the peak areas for all homologs in the chromatogram from the Ratio of Alkyl Components test |
| Mr | = | = molecular weight of a given homolog. The molecular weights of the C10, C12, C14, and C16 homologs are 312, 340, 368, and 396, respectively. |
Acceptance criteria
For labeled concentrations of NLT 1%:
95.0%–105.0%
For labeled concentrations less than 1%:
93.0%–107.0%
OTHER COMPONENTS
• Alcohol Content (if added)
Internal standard solution:
0.06 mL/mL of tertiary butyl alcohol in water
Alcohol stock solution:
0.03 mL/mL of C2H5OH in water, using USP Alcohol RS
Standard solutions:
Introduce 5 mL, 10 mL, and 20 mL, respectively, of Alcohol stock solution into three separate identical 50-mL volumetric flasks. To each flask, add a 5-mL portion of the Internal standard solution. Dilute with water to volume, and mix thoroughly. Standard solutions contain alcohol content of 0.003 mL/mL, 0.006 mL/mL, and 0.012 mL/mL, respectively.
Sample solution:
Weigh an appropriate amount of Benzalkonium Chloride Solution into a 50-mL volumetric flask, and pipet 5 mL of Internal standard solution. Dilute with water to volume, and mix thoroughly to obtain a solution containing an alcohol content of between 0.003 mL/mL and 0.012 mL/mL.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.25-mm × 30-cm glass or quartz capillary; 1.4-µm layer of phase G43
Temperature
Detector:
250
Injection port:
250
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 40 | — | 40 | 7 |
| 40 | 30 | 250 | 15 |
Run time:
29 min
Carrier gas:
Helium or nitrogen
Flow rate:
See the ramped flow program table below.
| Initial Flow (mL/min) |
Flow Ramp (mL/min2) |
Final Flow (mL/min) |
Hold Time at Final Flow (min) |
|---|---|---|---|
| 1 | — | 1 | 8 |
| 1 | 10 | 3 | 21 |
Injection size:
1 µL
Injection mode:
Split 75:1
System suitability
Sample:
Standard solution containing alcohol content of 0.006 mL/mL
[Note—The relative retention times for alcohol and tertiary butyl alcohol are 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 2.0, between alcohol and tertiary butyl alcohol
Relative standard deviation:
NMT 10%
Analysis
Samples:
Standard solutions and Sample solution
Plot the peak response ratios of the alcohol to tertiary butyl alcohol in the Standard solutions versus the content, in mL/mL, of alcohol, and draw the straight line best fitting the plotted points. From the graph obtained, determine the content, C, in mL/mL, of alcohol in the Sample solution. Calculate the percentage of alcohol in the portion of Benzalkonium Chloride Solution (v/v) taken:
Result = V × (C × D/W) × 100
| V | = | = volume of the Sample solution, 50 mL |
| D | = | = density of Benzalkonium Chloride Solution (g/mL) |
| W | = | = weight of Benzalkonium Chloride Solution taken to prepare the Sample solution (g) |
Acceptance criteria:
If present, between 95.0% and 105.0% of the labeled amount of C2H5OH
IMPURITIES
Organic Impurities
• Procedure 1: Limit of Amines and Amine Salts
Sample:
A quantity of Solution, equivalent to 5.0 g of benzalkonium chloride
Analysis and Acceptance criteria:
Dissolve the Sample with heating carefully e.g., on top of a steam bath with water as the steam source in 20 mL of a mixture of methanol and 1 N hydrochloric acid VS (97:3). [Note—However, the mixed solution must not reach the boiling point. ] Add 100 mL of isopropyl alcohol. Pass a stream of nitrogen slowly through the solution. Gradually add 12.0 mL of 0.1 N tetrabutylammonium hydroxide VS, while recording the potentiometric titration curve. If the curve shows two inflection points, the volume of titrant added between the two points is NMT 5.0 mL, corresponding to NMT 0.1 mmol/g of amines and amine salts. If the curve shows no point of inflection, the substance being examined does not comply with the test. If the curve shows one point of inflection, repeat the test, but add 3.0 mL of a 25.0 mg/mL solution of dimethyldecylamine in isopropyl alcohol before the titration. If after addition of 12.0 mL of the titrant, the titration curve shows only one point of inflection, the substance being examined does not comply with the test.
• Procedure 2: Limit of Benzyl Alcohol, Benzaldehyde, and (Chloromethyl)benzene
[Note—Prepare the solutions immediately before use. ]
Solution A:
Dissolve 1.09 g of sodium 1-hexanesulfonate and 6.9 g of monobasic sodium phosphate in water in a 1000-mL volumetric flask, adjust with phosphoric acid to a pH of 3.5, and dilute with water to volume.
Solution B:
Methanol
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 80 | 20 |
| 10 | 80 | 20 |
| 14 | 50 | 50 |
| 35 | 50 | 50 |
| 36 | 20 | 80 |
| 55 | 20 | 80 |
| 56 | 80 | 20 |
| 65 | 80 | 20 |
Standard solution A:
0.25 mg/mL of USP Benzyl Alcohol RS in methanol
Standard solution B:
0.075 mg/mL of USP Benzaldehyde RS in methanol
Standard solution C:
0.025 mg/mL of USP Benzyl Alcohol RS in methanol, prepared from Standard solution A and methanol
Sample solution:
Determine the density of the Benzalkonium Chloride Solution. Dilute a quantity of the Solution equivalent to 2.5 g of benzalkonium chloride with methanol to 50.0 mL. This solution contains 50 mg/mL of benzalkonium chloride.
Chromatographic system
Mode:
LC
Detector:
UV 210 nm for benzyl alcohol and (chloromethyl)benzene; UV 257 nm for benzaldehyde
Column:
4.6-mm × 15-cm analytical column; 5-µm packing L1
Column temperature:
30
Flow rate:
1.0 mL/min
Injection size:
20 µL
System suitability
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
[Note—See the relative retention times in the table below. ]
| Name | Relative Retention Time |
|---|---|
| Benzyl alcohol | 1.0 |
| Benzaldehyde | 1.3 |
| (Chloromethyl)benzene | 2.4 |
Suitability requirements
Relative standard deviation:
NMT 5.0% for the benzyl alcohol peak, Standard solution A
Signal-to-noise ratio:
NLT 10 for the principal peak in the chromatogram, Standard solution C
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
To calculate the content of (chloromethyl)benzene, multiply the peak area of (chloromethyl)benzene by 1.3. [Note—The correction factor is used due to baseline shift. ]
Acceptance criteria
Benzyl alcohol:
The response of the benzyl alcohol peak from the Sample solution is NMT that of the benzyl alcohol peak from Standard solution A, corresponding to NMT 0.5%.
Benzaldehyde:
The response of the benzaldehyde peak from the Sample solution is NMT that of the benzaldehyde peak from Standard solution B, corresponding to NMT 0.15%.
(Chloromethyl)benzene:
The response of the (chloromethyl)benzene peak from the Sample solution is NMT 0.1 times that of the principal peak in the chromatogram from Standard solution A, corresponding to NMT 0.05%.
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
Solution containing less than 5.0% of benzalkonium chloride meets the requirements of the test for absence of Pseudomonas aeruginosa.
:
Solution containing less than 5.0% of benzalkonium chloride meets the requirements of the test for absence of Pseudomonas aeruginosa.
• Acidity or Alkalinity
Sample solution:
An appropriate quantity of the Solution, equivalent to 500 mg of benzalkonium chloride solid, prepared from Benzalkonium Chloride Solution and carbon dioxide-free water
Analysis:
To the Sample solution add 0.1 mL of bromocresol purple TS.
Acceptance criteria:
NMT 0.1 mL of 0.1 N hydrochloric acid or 0.1 N sodium hydroxide is required to change the color of the indicator.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and prevent contact with metals.
• Labeling:
Label it to indicate the concentration of benzalkonium chloride, and to indicate the name and quantity of coloring agent added. The labeling also indicates the concentration of alcohol added.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1710
Pharmacopeial Forum: Volume No. 35(6) Page 1499