Bentonite
Bentonite

 [1302-78-9].
[1302-78-9].
(ben' ton ite).
Bentonite
DEFINITION
Bentonite is a native, colloidal, hydrated aluminum silicate.
IDENTIFICATION
• A. X-Ray Diffraction  941
941
Sample A:
Add 2 g in small portions to 100 mL of water, with intense agitation. Allow to stand for 12 h to ensure complete hydration. Place 2 mL of the mixture so obtained on a suitable glass slide, and allow to air-dry at room temperature to produce an oriented film. Place the slide in a vacuum desiccator over a free surface of ethylene glycol. Evacuate the desiccator, and close the stopcock so that the ethylene glycol saturates the desiccator chamber. Allow to stand for 12 h.
Sample B:
Prepare a random powder specimen of Bentonite.
Analysis
Samples:
Sample A and Sample B
Record the X-Ray diffraction pattern of the samples, and determine the d values
Acceptance criteria:
The largest peak in the pattern of Sample A corresponds to a d value between 15.0 and 17.2 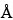 . The major peak in the region between 1.48 and 1.54
. The major peak in the region between 1.48 and 1.54 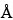 from the pattern of Sample B is 1.492 and 1.504
from the pattern of Sample B is 1.492 and 1.504 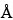 .
.
IMPURITIES
• Arsenic, Method I  211
211
Test preparation:
Transfer 8.0 g to a 250-mL beaker containing 100 mL of dilute hydrochloric acid (1 in 25), mix, and cover with a watch glass. Boil gently, with occasional stirring, for 15 min without allowing excessive foaming. Pass the hot supernatant through a rapid-flow filter paper into a 200-mL volumetric flask, and wash with four 25-mL portions of hot dilute hydrochloric acid (1 in 25), collecting the washings in the volumetric flask. Cool the combined filtrates to room temperature, and add dilute hydrochloric acid (1 in 25) to volume. Transfer 25 mL of this solution to a generator flask, and dilute with water to 35 mL.
Standard preparation:
Transfer 5 mL of Standard Arsenic Solution (5 µg) to a generator flask, and dilute with water to 35 mL.
Analysis:
Proceed as directed in the chapter, determining the absorbances.
Acceptance criteria:
The absorbance due to any red color from the Test preparation does not exceed that produced by the Standard preparation (NMT 5 ppm).
• Lead
[Note—The Standard solution and the Sample solution may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument. It may be necessary to correct the value obtained for the Sample solution for interference from the sample specimen matrix. ]
Standard solution:
On the day of use, dilute 3.0 mL of Lead Nitrate Stock Solution (see Heavy Metals  231
231 ) with water to 100 mL. Each mL of the Standard solution contains the equivalent of 3 µg of lead.
) with water to 100 mL. Each mL of the Standard solution contains the equivalent of 3 µg of lead.
Sample solution:
Transfer 3.75 g to a 250-mL beaker containing 100 mL of dilute hydrochloric acid (1 in 25), stir, and cover with a watch glass. Boil for 15 min, then cool to room temperature, and pass through a rapid-flow filter paper into a 400-mL beaker. Wash the filter with four 25-mL portions of hot water, collecting the washings in the 400-mL beaker. Concentrate the combined extracts by gentle boiling to approximately 20 mL. If a precipitate appears, add 2–3 drops of nitric acid, heat to boiling, and cool to room temperature. Pass the concentrated extracts through a rapid-flow filter paper into a 50-mL volumetric flask. Transfer the remaining contents of the 400-mL beaker through the filter paper and into the flask with water. Dilute with water to volume.
Instrumental conditions
Mode:
Atomic absorption spectrophotometry
Analytical wavelength:
283.3 nm
Lamp:
Lead hollow cathode with deuterium arc background correction
Flame:
Air and acetylene
Analysis:
Determine the absorbances of the Standard solution and the Sample solution.
Acceptance criteria:
The absorbance of the Sample solution is NMT that of the Standard solution (40 ppm).
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
Meets the requirements of the test for absence of Escherichia coli
:
Meets the requirements of the test for absence of Escherichia coli
• pH  791
791 :
9.5–10.5. Disperse 4.0 g in 200 mL of water, mixing vigorously to facilitate wetting.
:
9.5–10.5. Disperse 4.0 g in 200 mL of water, mixing vigorously to facilitate wetting.
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 2 h: it loses 5.0%–8.0% of its weight.
for 2 h: it loses 5.0%–8.0% of its weight.
• Gel Formation:
Mix 6 g with 300 mg of magnesium oxide. Add the mixture, in several divided portions, to 200 mL of water contained in a blender of approximately 500-mL capacity. Blend thoroughly for 5 min at high speed, transfer 100 mL of the mixture to a 100-mL graduated cylinder, and allow to remain undisturbed for 24 h: NMT 2 mL of supernatant appears on the surface.
• Swelling Power:
To 100 mL of water contained in a glass-stoppered cylinder of 100-mL capacity add 2 g in portions, dropping it upon the surface of the water, and allow each portion to settle before adding the next. The mass at the bottom gradually swells until it occupies an apparent volume of NLT 24 mL at the end of a 2-h period.
• Fineness of Powder:
Sprinkle 2 g on 20 mL of water contained in a mortar. Allow to swell, disperse evenly with a pestle, and dilute with water to 100 mL. Pour the suspension through a No. 200 standard sieve, and wash the sieve thoroughly with water. No grit is felt when the fingers are rubbed over the wire mesh of the sieve.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Label it to indicate that absorption of atmospheric moisture should be avoided following the opening of the original package, preferably by storage of the remainder of the contents in a tight container.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 1705