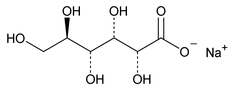
Sodium Gluconate
(soe' dee um gloo' koe nate).
DEFINITION
Sodium Gluconate contains NLT 98.0% and NMT 102.0% of C6H11NaO7.
IDENTIFICATION
• A. Identification Tests—General, Sodium  191
191
Sample solution:
1 in 20
Acceptance criteria:
Meets the requirements
• B. Thin-Layer Chromatography
Standard solution:
10 mg/mL of USP Potassium Gluconate RS in water. [Note—Heat in a water bath at 60 if necessary to dissolve. ]
if necessary to dissolve. ]
Sample solution:
10 mg/mL of Sodium Gluconate in water. [Note—Heat in a water bath at 60 if necessary to dissolve. ]
if necessary to dissolve. ]
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic silica gel
Application volume:
5 µL
Developing solvent system:
Alcohol, ethyl acetate, ammonium hydroxide, and water (50:10:10:30)
Spray reagent:
Dissolve 2.5 g of ammonium molybdate in 50 mL of 2 N sulfuric acid in a 100-mL volumetric flask, add 1.0 g of ceric sulfate, swirl to dissolve, and dilute with 2 N sulfuric acid to volume.
Analysis
Samples:
Standard solution and Sample solution
Develop in the Developing solvent system until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the chamber, and dry at 110 for 20 min. Allow to cool and spray with the Spray reagent. Heat the plate at 110
for 20 min. Allow to cool and spray with the Spray reagent. Heat the plate at 110 for 10 min.
for 10 min.
Acceptance criteria:
The principal spot from the Sample solution corresponds in color, size, and RF value to that from the Standard solution.
ASSAY
• Procedure
Sample:
150 mg
Analysis:
Transfer the Sample to a suitable conical flask and dissolve in 75 mL of glacial acetic acid, warming if necessary to dissolve. Cool, add quinaldine red TS, and titrate with 0.1 N perchloric acid VS to a colorless endpoint. Each mL of 0.1 N perchloric acid is equivalent to 21.81 mg of C6H11NaO7.
Acceptance criteria:
98.0%–102.0%
IMPURITIES
• Chloride and Sulfate, Chloride  221
221
Sample:
1.0 g
Acceptance criteria:
The Sample shows no more chloride than corresponds to 1 mL of 0.020 N hydrochloric acid (NMT 0.07%).
• Chloride and Sulfate, Sulfate  221
221
Sample:
2.0 g
Acceptance criteria:
The Sample, dissolved in boiling water, shows no more sulfate than corresponds to 1 mL of 0.020 N sulfuric acid (NMT 0.05%).
• Heavy Metals, Method I  231
231
Test preparation:
Dissolve 1.0 g in 10 mL of water, add 6 mL of 3 N hydrochloric acid, and dilute with water to 25 mL.
Acceptance criteria:
NMT 20 ppm
• Reducing Substances
Sample:
1.0 g
Analysis:
Transfer the Sample to a 250-mL conical flask, dissolve in 10 mL of water, and add 25 mL of alkaline cupric citrate TS. Cover the flask, boil gently for 5 min, accurately timed, and cool rapidly to room temperature. Add 25 mL of 0.6 N acetic acid, 10.0 mL of 0.1 N iodine VS, and 10 mL of 3 N hydrochloric acid, and titrate with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, omitting the specimen, and note the difference in volumes required (see Titrimetry  541
541 ). Each mL of the difference in volume of 0.1 N sodium thiosulfate consumed is equivalent to 2.7 mg of reducing substances (as dextrose).
). Each mL of the difference in volume of 0.1 N sodium thiosulfate consumed is equivalent to 2.7 mg of reducing substances (as dextrose).
Acceptance criteria:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4661