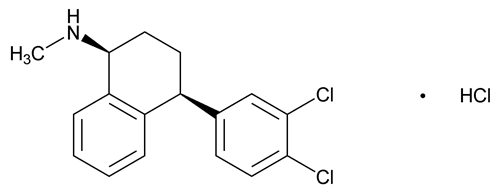
Sertraline Hydrochloride
C17H17Cl2N·HCl 342.69
1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-, hydrochloride, (1S-cis);
(1S,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride

 [79559-97-0].
[79559-97-0].
(ser' tra leen hye'' droe klor' ide).
C17H17Cl2N·HCl 342.69
1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-, hydrochloride, (1S-cis);
(1S,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride
DEFINITION
Sertraline Hydrochloride contains NLT 97.0% and NMT 102.0% of C17H17Cl2N·HCl, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of sertraline hydrochloride from the System suitability solution, as obtained in the test for Limit of (R,R) sertraline hydrochloride.
• C. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
Sample solution:
A solution (1 in 10) in a mixture of alcohol and water (1:1)
ASSAY
• Procedure
Buffer:
5.75 g/L of monobasic ammonium phosphate in water. Adjust with phosphoric acid to a pH 4.2.
Mobile phase:
Methanol and Buffer (12:13)
Standard solution:
0.04 mg/mL of USP Sertraline Hydrochloride RS in Mobile phase
Sample solution:
0.04 mg/mL of Sertraline Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.0-mm × 25-cm; 5-µm packing L45
Column temperature:
30
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.3
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C17H17Cl2N·HCl in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Sertraline Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
97.0%–102.0% on the anydrous basis
IMPURITIES
SPECIFIC TESTS
• Water Determination, Method Ia  921
921 :
NMT 0.50%
:
NMT 0.50%
• Limit of (R,R) Sertraline Hydrochloride
Mobile phase:
Hexane, 2-propanol, and diethylamine (960:40:1.5)
System suitability solution:
Transfer 10 mg of USP Sertraline Hydrochloride Racemic Mixture RS into a 20-mL volumetric flask. Add 4 mL of diluted ammonia water (1 in 10) and 10 mL of hexane. Shake well until the organic phase is clear. Wait for phase separation, transfer about 2.0 mL from the top layer into a 20-mL volumetric flask, and dilute with hexane to volume.
Standard solution:
Transfer 10 mg of USP Sertraline Hydrochloride RS into a 20-mL volumetric flask. Add 4 mL of diluted ammonia water (1 in 10) and 10 mL of hexane. Shake well until the organic phase is clear. Wait for phase separation, transfer 1.0 mL from the top layer into a 10-mL volumetric flask, and dilute with hexane to volume. Further dilute quantitatively and stepwise, if necessary, to obtain a solution having a known concentration of 0.01 mg/mL.
Sample solution:
Transfer 20 mg of Sertraline Hydrochloride to a 20-mL volumetric flask. Add 4 mL of diluted ammonia water (1 in 10) and 10 mL of hexane. Shake well until the organic phase is clear. Wait for phase separation, and use the top layer.
Chromatographic system
Mode:
LC
Detector:
UV 235 nm
Column:
4.6-mm × 25-cm; 5-µm packing L40
Column temperature:
5
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for (R,R) sertraline hydrochloride and sertraline hydrochloride are about 1.16 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 2.8 between sertraline hydrochloride and (R,R) sertraline hydrochloride
Relative standard deviation:
NMT 10.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of (R,R) sertraline hydrochloride in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for (R,R) sertraline hydrochloride from the Sample solution |
| rS | = | = peak response for Sertraline Hydrochloride from the Standard solution |
| CS | = | = concentration of USP Sertraline Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 1.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers at a temperature not exceeding 40 .
.
• USP Reference Standards  11
11
USP Sertraline Hydrochloride Racemic Mixture RS
(1RS,4RS)-4-(3,4-Dichlorophenyl)-N-methyl-1,2,3,4-tetrahydro-1-naphthylamine hydrochloride.
C17H17CI2·HCI 342.69
(1RS,4RS)-4-(3,4-Dichlorophenyl)-N-methyl-1,2,3,4-tetrahydro-1-naphthylamine hydrochloride.
C17H17CI2·HCI 342.69
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4629
Pharmacopeial Forum: Volume No. 34(5) Page 1189