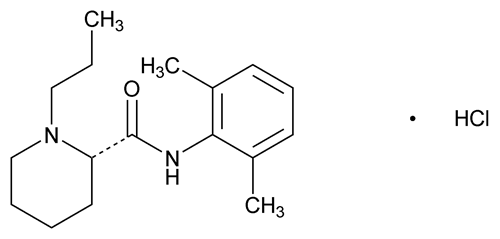
Ropivacaine Hydrochloride
C17H26N2O ·HCl ·H2O 328.89
(S)-(–)-1-Propylpiperidine-2-carboxylic acid (2,6-dimethylphenyl)amide hydrochloride monohydrate;
(S)-(–)-1-Propyl-2¢,6¢-pipecoloxylidine hydrochloride monohydrate

 [132112-35-7].
[132112-35-7].
(roe piv' a kane hye'' droe klor' ide).
C17H26N2O ·HCl ·H2O 328.89
(S)-(–)-1-Propylpiperidine-2-carboxylic acid (2,6-dimethylphenyl)amide hydrochloride monohydrate;
(S)-(–)-1-Propyl-2¢,6¢-pipecoloxylidine hydrochloride monohydrate
DEFINITION
Ropivacaine Hydrochloride contains NLT 98.5% and NMT 101.0% of C17H26N2O·HCl, calculated on the anhydrous basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191
Sample solution:
10 mg/mL
ASSAY
• Procedure
Sample solution:
Dissolve 1000 mg of Ropivacaine Hydrochloride in 10 mL of water and 40 mL of alcohol. Add 1.0 mL of 1 N hydrochloric acid.
Analysis:
Titrate with 1 N sodium hydroxide VS. Two equivalence points are obtained; the difference in titrant volume corresponds to the amount of ropivacaine hydrochloride (see Titrimetry  541
541 ). Each mL of 1 N sodium hydroxide is equivalent to 310.9 mg of anhydrous ropivacaine hydrochloride (C17H26N2O·HCl).
). Each mL of 1 N sodium hydroxide is equivalent to 310.9 mg of anhydrous ropivacaine hydrochloride (C17H26N2O·HCl).
Acceptance criteria:
98.5%–101.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals  231
231
pH 3.5 acetate buffer and Standard lead solution:
Prepare as directed under Heavy Metals  231
231 .
.
Dilute lead standard solution:
Dilute 10.0 mL of the Standard lead solution with water to 100 mL. Each mL of Dilute lead standard solution contains the equivalent of 1 µg of lead.
0.25 M Sodium sulfide solution:
Dissolve 6.0 g of sodium sulfide in 40 g of glycerol, then dilute with water to 100 mL. Filter using a cotton pad, and store in a glass container protected from light.
Sample stock solution:
Prepare as directed under Heavy Metals  231
231 , Method II, using 3.97–4.00 g of Ropivacaine Hydrochloride.
, Method II, using 3.97–4.00 g of Ropivacaine Hydrochloride.
Standard solution:
Combine 10.0 mL of the Dilute lead standard solution with 2 mL of the Sample stock solution and 2 mL of pH 3.5 acetate buffer.
Sample solution:
Combine 12 mL of the Sample stock solution with 2 mL of pH 3.5 acetate buffer.
Blank:
Combine 10 mL of water, 2 mL of pH 3.5 acetate buffer, and 2 mL of the Sample stock solution.
Analysis:
Transfer the Blank to a color-comparison tube. Transfer the Standard solution and the Sample solution to individual color-comparison tubes each containing 1 drop of 0.25 M sodium sulfide solution. After 1 min, compare the colors, viewing downward over a white surface.
Acceptance criteria:
The Standard solution shows a slight brown color compared to the Blank. The Sample solution is not darker than the Standard solution (NMT 10 ppm).
Organic Impurities
• Procedure 1
Buffer solution:
Combine 1.3 mL of monobasic sodium phosphate solution (138 g/L) and 32.5 mL of disodium hydrogen phosphate dihydrate solution (89 g/L), and dilute with water to 1 L. The pH of this solution is 8.0. Make adjustments if necessary.
Mobile phase:
Acetonitrile and Buffer solution (1:1)
System suitability solution:
10 µg/mL of each of USP Ropivacaine Hydrochloride RS and USP Bupivacaine Hydrochloride RS in Mobile phase
Sample solution 1:
2.75 mg/mL of Ropivacaine Hydrochloride in Mobile phase
Sample solution 2:
2.75 µg/mL of Ropivacaine Hydrochloride from Sample solution 1 diluted with Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 240 nm
Column:
3.9-mm × 15-cm; 4-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
[Note—Check the stability of the baseline by injecting Mobile phase. Run the chromatogram for at least 15 min. ]
Samples:
System suitability solution and Sample solution 2
[Note—The relative retention times for ropivacaine and bupivacaine are about 1.0 and 1.6, respectively. ]
Suitability requirements
Resolution:
NLT 6 between ropivacaine and bupivacaine, System suitability solution
Signal-to-noise ratio:
NLT 10 for ropivacaine, Sample solution 2
Analysis
Samples:
System suitability solution and Sample solution 1
Calculate the percentage of each impurity in the portion of Ropivacaine Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rT | = | = sum of all the peak responses from the Sample solution |
Acceptance criteria
Bupivacaine:
NMT 0.2%
Any other individual impurity:
Less than 0.1%
Total impurities:
NMT 0.5%
• Procedure 2: Limit of Ropivacaine Related Compound A
Buffer solution, Mobile phase, and Chromatographic system:
Prepare as directed in Procedure 1.
Standard solution:
0.13 µg/mL of USP Ropivacaine Related Compound A RS in Mobile phase
Sample solution:
10 mg/mL of Ropivacaine Hydrochloride in Mobile phase
System suitability
Sample:
Standard solution
Suitability requirements
Signal-to-noise ratio:
NLT 10 for ropivacaine related compound A
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria:
The response for any peak corresponding to ropivacaine related compound A (2,6-dimethylaniline) in the Sample solution is not greater than the response of the major peak in the Standard solution (NMT 10 ppm).
• Procedure 3: Enantiomeric Purity
Background electrolyte solution:
9.31–10.29 mg/mL of phosphoric acid in water. The pH is between 2.9 and 3.1. If necessary, adjust the pH with triethanolamine to 2.9–3.1.
Run buffer:
13.3 mg/mL of heptakis-(2,6-di-O-methyl)- -cyclodextrin in Background electrolyte solution. [Note—This solution is freshly prepared and passed through a 0.45-µm filter. ]
-cyclodextrin in Background electrolyte solution. [Note—This solution is freshly prepared and passed through a 0.45-µm filter. ]
System suitability solution:
15 µg/mL of each USP Ropivacaine Hydrochloride RS and USP Ropivacaine Related Compound B RS in water
Sample solution 1:
2 mg/mL of Ropivacaine Hydrochloride in water
Sample solution 2:
0.01 mg/mL of ropivacaine hydrochloride from Sample solution 1 diluted with water
Capillary rinsing procedure:
Use separate Run buffer vials for capillary rinse and sample analysis. Rinse the capillary with water for 1 min, with 0.1 N sodium hydroxide for 10 min, and with water for 3 min. If a new or dry capillary is being used, increase the sodium hydroxide rinse time to 30 min. Rinse the capillary between injections as follows: water for 1 min, 0.1 N sodium hydroxide for 4 min, and water for 1 min, then Run buffer for 4 min. Rinse times are based on a rinse pressure of 1 bar.
Capillary electrophoresis system
Detector:
UV 206 nm
Column:
50-µm × 72-cm fused silica
Column temperature:
30
Applied voltage:
375 V/cm
Initial ramping:
500 V/s, positive polarity, and a resulting current of 40–45 µA
Injection size:
Equal volumes
System suitability
Samples:
System suitability solution and Sample solution 2
[Note—The relative migration times for ropivacaine related compound B (R enantiomer) and ropivacaine (S enantiomer) are about 0.96 and 1.0, respectively. ]
Suitability requirements
Signal-to-noise ratio:
NLT 10, Sample solution 2
Resolution:
NLT 3.7 between ropivacaine related compound B and ropivacaine, System suitability solution
[Note—The analysis run time is about 30 min. If needed, increase the resolution by increasing the concentration of heptakis-(2,6-di-O-methyl)- -cyclodextrin or by lowering the system temperature. ]
-cyclodextrin or by lowering the system temperature. ]
Analysis
Samples:
Run buffer, water, and Sample solution 1
Inject Run buffer and water to ensure there are no interfering peaks (50 mbar for 5.0 s followed by injection of Run buffer at 50 mbar for 1.0 s). Inject Sample solution 1 into the electrophoresis system, record the electropherograms, and measure the peak responses for ropivacaine and ropivacaine related compound B.
Calculate the percentage of ropivacaine related compound B in the portion of Ropivacaine Hydrochloride taken:
Result = (rR/MR)/(rS/MS) × 100
| rR | = | = peak response of ropivacaine related compound B from Sample solution 1 |
| MR | = | = migration time of ropivacaine related compound B (min) |
| rS | = | = peak response of ropivacaine from Sample solution 1 |
| MS | = | = migration time of ropivacaine (min) |
[Note—After the analysis, rinse the capillary for 10 min with 0.1 N sodium hydroxide, then for 10 min with water. Dry the capillary before storage. ]
Acceptance criteria:
NMT 0.5% of ropivacaine related compound B
SPECIFIC TESTS
• Bacterial Endotoxins Test  85
85 :
The level of bacterial endotoxins is such that the requirements under the relevant dosage form monograph(s) in which Ropivacaine Hydrochloride is used can be met. Where the label states that Ropivacaine Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirements under the relevant dosage form monograph(s) in which Ropivacaine Hydrochloride is used can be met.
:
The level of bacterial endotoxins is such that the requirements under the relevant dosage form monograph(s) in which Ropivacaine Hydrochloride is used can be met. Where the label states that Ropivacaine Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirements under the relevant dosage form monograph(s) in which Ropivacaine Hydrochloride is used can be met.
• Color
Sample solution:
Transfer an aliquot of Ropivacaine Hydrochloride, 480–500 mg, into a 25-mL volumetric flask, and dissolve in and dilute with water to volume. Pass the solution through a 5-µm polyvinylidene filter (PVDF).
Spectrometric conditions
Mode:
UV-Vis
Analytical wavelengths:
405 and 436 nm
Cell:
5 cm
Analysis
Sample:
Sample solution
Immediately measure the absorbance of the Sample solution, using water as the reference.
Acceptance criteria:
NMT 0.030 at 405 nm, and NMT 0.025 at 436 nm
• Clarity
Hydrazine sulfate solution:
10 mg/mL of hydrazine sulfate in water. Allow to stand 4–6 h.
Hexamethylenetetramine solution:
Transfer 2.5 g of hexamethylenetetramine to a 100-mL glass-stoppered flask, and dissolve in 25 mL of water.
Opalescence standard stock suspension:
To the flask containing the Hexamethylenetetramine solution, add 25.0 mL of Hydrazine sulfate solution, mix, and allow to stand for 24 h. This suspension is stable for up to 2 months when stored in a glass container free from surface defects. The suspension must not adhere to the flask and must be well mixed before use.
Opalescence standard suspension:
Dilute 15.0 mL of the Opalescence standard stock suspension with water to 1000 mL. This suspension should be freshly prepared and may be stored for NMT 24 h.
Standard suspension 1:
Opalescence standard suspension and water (5:95). Shake before use.
Standard suspension 2:
Opalescence standard suspension and water (10:90). Shake before use.
Sample solution:
480–500 mg of Ropivacaine Hydrochloride in a 25-mL volumetric flask. Dilute with water to volume.
Analysis:
Samples:
Standard suspension 1, Standard suspension 2, and Sample solution
Use identical tubes of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm. The depth of the layer is 40 mm. Compare the solutions in diffused daylight 5 min after the preparation of Standard suspension 1 and Standard suspension 2, viewing vertically against a black background. The diffusion of light must be such that Standard suspension 1 can readily be distinguished from water, and Standard suspension 2 can readily be distinguished from Standard suspension 1.
Acceptance criteria:
The Sample solution is considered clear if its clarity is the same as that of water or if its opalescence is not more pronounced than that of Standard suspension 1.
• pH  791
791 :
4.5–6.0, in a solution (10 mg/mL)
:
4.5–6.0, in a solution (10 mg/mL)
• Water Determination, Method Ia  921
921 :
5.0%–6.0%. Perform the determination on 0.0900–0.1100 g of sample.
:
5.0%–6.0%. Perform the determination on 0.0900–0.1100 g of sample.
• Sterility Tests  71
71 :
Where the label states that Ropivacaine Hydrochloride is sterile, it meets the requirements.
:
Where the label states that Ropivacaine Hydrochloride is sterile, it meets the requirements.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. Store at room temperature.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
• USP Reference Standards  11
11
USP Endotoxin RS
USP Ropivacaine Related Compound A RS
2,6-Dimethylaniline hydrochloride.
C8H12ClN 157.64

 [CAS-21436-98-6].
[CAS-21436-98-6].
2,6-Dimethylaniline hydrochloride.
C8H12ClN 157.64
USP Ropivacaine Related Compound B RS
(R)-ropivacaine hydrochloride monohydrate; (R)-(–)-1-propylpiperidene-2-carboxylic acid (2,6-dimethylphenyl)-amide hydrochloride monohydrate.
C17H26N2O 328.89
(R)-ropivacaine hydrochloride monohydrate; (R)-(–)-1-propylpiperidene-2-carboxylic acid (2,6-dimethylphenyl)-amide hydrochloride monohydrate.
C17H26N2O 328.89
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4589
Pharmacopeial Forum: Volume No. 36(1) Page 135