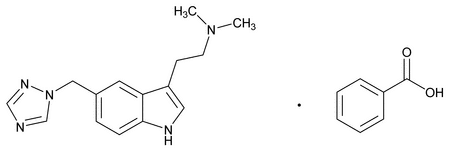
Rizatriptan Benzoate
C22H25N5O2 391.47
1H-Indole-3-ethanamine, N,N-dimethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-, monobenzoate;
3-[2-(Dimethylamino)ethyl]-5-(1H-1,2,4-triazol-1-ylmethyl)indole monobenzoate

 [145202-66-0].
[145202-66-0].
(rye'' za trip' tan ben' zoe ate).
C22H25N5O2 391.47
1H-Indole-3-ethanamine, N,N-dimethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-, monobenzoate;
3-[2-(Dimethylamino)ethyl]-5-(1H-1,2,4-triazol-1-ylmethyl)indole monobenzoate
DEFINITION
Rizatriptan Benzoate contains NLT 98.0% and NMT 102.0% of C22H25N5O2, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197
197
[Note—Methods described under Infrared Absorption 197K, 197M, or 197A may be used. If the spectra obtained show differences, dissolve the substance to be examined and the Reference Standard separately in methanol, evaporate to dryness, and record the new spectra using the residues. ]
• B.
The retention times of the major peaks of the Sample solution correspond to those of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note—Use silanized autosampler vials and freshly prepared solutions. ]
Solution A:
Add 1.0 mL of trifluroacetic acid to 1 L of a solution of acetonitrile and water (4:21), and mix.
Solution B:
Acetonitrile and trifluroacetic acid (1000:1)
Standard solution:
1 mg/mL of USP Rizatriptan Benzoate RS in Solution A
Sample solution:
1 mg/mL of Rizatriptan Benzoate in Solution A
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 8.0 | 100 | 0 |
| 17.0 | 70 | 30 |
| 20.0 | 70 | 30 |
| 20.1 | 100 | 0 |
| 23.0 | 100 | 0 |
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.6-mm × 25-cm; 5-µm packing L11
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for rizatriptan and benzoic acid are 1.0 and about 2.1, respectively. ]
Suitability requirements
Tailing factor:
NMT 3.5 for rizatriptan
Relative standard deviation:
NMT 0.73% for the rizatriptan peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C22H25N5O2 in the portion of Rizatriptan Benzoate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of rizatriptan from the Sample solution |
| rS | = | = peak response of rizatriptan from the Standard solution |
| CS | = | = concentration of USP Rizatriptan Benzoate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Rizatriptan Benzoate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals  231
231 :
NMT 10 ppm
:
NMT 10 ppm
Sample solution:
Dissolve 0.50 g of Rizatriptan Benzoate in 20 mL of water.
Reference solution:
Dilute 0.5 mL of the Standard Lead Solution, prepared as directed under Heavy Metals  231
231 , with water to 20 mL.
, with water to 20 mL.
Blank solution:
20 mL of water
Analysis
Samples:
Reference solution, Sample solution, and Blank solution
To each solution add 2 mL of pH 3.5 Acetate Buffer, prepared as directed under Heavy Metals  231
231 . Add each of the resulting solutions to 1.2 mL of thioacetamide–glycerin base TS. Mix immediately, and allow to stand for 2 min. Pass the solutions through a membrane filter of 0.45-µm pore size. Compare the spots on the filters obtained from the different solutions: the brownish-black color of the spot resulting from the Sample solution is not more intense than that of the spot resulting from the Reference solution. The test is invalid if the Reference solution does not show a brownish-black color compared to the Blank solution.
. Add each of the resulting solutions to 1.2 mL of thioacetamide–glycerin base TS. Mix immediately, and allow to stand for 2 min. Pass the solutions through a membrane filter of 0.45-µm pore size. Compare the spots on the filters obtained from the different solutions: the brownish-black color of the spot resulting from the Sample solution is not more intense than that of the spot resulting from the Reference solution. The test is invalid if the Reference solution does not show a brownish-black color compared to the Blank solution.
Organic Impurities
• Procedure
Solution A, Solution B, and Mobile phase:
Proceed as directed in the Assay.
Sample solution:
1 mg/mL of Rizatriptan Benzoate in Solution A
System suitability solution:
1 mg/mL of USP Rizatriptan Benzoate System Suitability Mixture RS in Solution A
Sensitivity solution:
0.5 µg/mL of Rizatriptan Benzoate obtained by suitable dilution of the Sample solution with Solution A
Chromatographic system:
Prepare as directed in the Assay.
System suitability
Sample:
System suitability solution and Sensitivity solution
[Note—The relative retention times for rizatriptan, rizatriptan impurity C, and benzoic acid are 1.0, about 1.3, and about 2.1, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between rizatriptan and rizatriptan impurity C, System suitability solution
Signal-to-noise ratio:
NLT 10 for the rizatriptain peak, Sensitivity solution
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Rizatriptan Benzoate taken:
Result = [rU/(rT  rBA)] × 100
rBA)] × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of the areas of all the peaks from the Sample solution |
| rBA | = | = area of the benzoic acid peak from the Sample solution |
Acceptance criteria
Any individual impurity:
NMT 0.10%
Total:
NMT 0.3%. [Note—Disregard any impurity that is less than 0.05%. ]
SPECIFIC TESTS
• Water Determination, Method Ia  921
921 :
NMT 0.5%
:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Store in well-closed containers at room temperature.
• USP Reference Standards  11
11
USP Rizatriptan Benzoate RS
USP Rizatriptan Benzoate System Suitability Mixture RS. Mixture of rizatriptan benzoate and at least 0.1% of rizatriptan impurity C. Rizatriptan impurity C is 2-{5-[(1H-1,2,4-triazol-1-yl)methyl]-1H-indol-2-yl}-N,N-dimethylethanamine.
(C15H19N5 269.34)
(C15H19N5 269.34)
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4583
Pharmacopeial Forum: Volume No. 36(1) Page 132