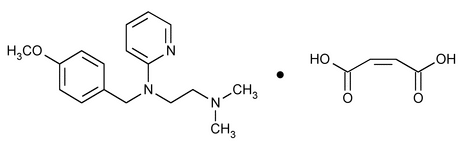
Pyrilamine Maleate
(pir il' a meen mal' ee ate).
1,2-Ethanediamine, N-[(4-methoxyphenyl)methyl]-N¢,N¢-dimethyl-N-2-pyridinyl-, (Z)-2-butenedioate (1:1).
2-[[2-(Dimethylamino)ethyl](p-methoxybenzyl)amino]pyridine maleate (1:1)
» Pyrilamine Maleate, dried in vacuum over phosphorus pentoxide for 5 hours, contains not less than 98.0 percent and not more than 100.5 percent of C17H23N3O· C4H4O4.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
10 µg per mL.
Medium:
0.5 N sulfuric acid.
Absorptivities at 236 nm and 312 nm, calculated on the dried basis, do not differ by more than 3.0%.
Melting range, Class I  741
741 :
between 99
:
between 99 and 103
and 103 .
.
Loss on drying  731
731 —
Dry it in vacuum over phosphorus pentoxide for 5 hours: it loses not more than 0.5% of its weight.
—
Dry it in vacuum over phosphorus pentoxide for 5 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Related compounds—
test
1—
Standard solution—
Dissolve an accurately weighed quantity of USP Pyrilamine Maleate RS in a mixture of methanol and ammonium hydroxide (200:1) to obtain a solution having a known concentration of about 0.4 mg per mL. Quantitatively dilute this solution with the mixture of methanol and ammonium hydroxide (200:1) to obtain Standard solutions A, B, and C having the following compositions:
| Standard solution |
Dilution | Concentration (mg of RS per mL) | Percentage (%, for comparison with test specimen) |
|---|---|---|---|
| A | (1 in 4) | 0.1 | 0.5 |
| B | (3 in 20) | 0.06 | 0.3 |
| C | (1 in 20) | 0.02 | 0.1 |
Test solution—
Dissolve an accurately weighed quantity of Pyrilamine Maleate in a mixture of methanol and ammonium hydroxide (200:1) to obtain a solution having a known concentration of about 20 mg per mL.
Eluant:
ethyl acetate, diethylamine, n-hexane, and methanol (93:7:1:1).
Procedure—
Apply separately 10 µL of the Test solution and 10 µL of each of the three Standard solutions to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. [note—The plate has been prewashed for 2 hours with Eluant and dried. ] Allow the spots on the plate to dry. Place the plate in a chromatographic chamber and develop the chromatograms in Eluant until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and air-dry the plate. View the plate under short-wavelength UV light and compare the intensities of any secondary spots from the chromatogram of the Test solution with those of the principal spots from the chromatograms of the Standard solutions. No secondary spot from the chromatogram of the Test solution is larger or more intense than the principal spot from Standard solution A (0.5%), and the sum of the intensities of all secondary spots from the Test solution corresponds to not more than 1.0%.
) coated with a 0.25-mm layer of chromatographic silica gel mixture. [note—The plate has been prewashed for 2 hours with Eluant and dried. ] Allow the spots on the plate to dry. Place the plate in a chromatographic chamber and develop the chromatograms in Eluant until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and air-dry the plate. View the plate under short-wavelength UV light and compare the intensities of any secondary spots from the chromatogram of the Test solution with those of the principal spots from the chromatograms of the Standard solutions. No secondary spot from the chromatogram of the Test solution is larger or more intense than the principal spot from Standard solution A (0.5%), and the sum of the intensities of all secondary spots from the Test solution corresponds to not more than 1.0%.
test
2—
Mobile phase—
Prepare a filtered and degassed mixture of 0.01 M ammonium acetate, methanol, and triethylamine (40:60:0.1). Make adjustments, if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve an accurately weighed quantity of USP Pyrilamine Maleate RS in Mobile phase to obtain a solution having a known concentration of about 0.5 µg per mL.
Test solution—
Transfer about 50 mg of Pyrilamine Maleate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.0-mm × 30-cm column that contains packing L11. The flow rate is about 1.0 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.0-mm × 30-cm column that contains packing L11. The flow rate is about 1.0 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Inject a volume (about 20 µL) of the Test solution into the chromatograph, run the chromatograph for 25 minutes, record the chromatograms, and measure the peak area responses, but do not measure the maleate peak area response, which elutes near the void volume. Calculate the percentage of each impurity in the portion of pyrilamine taken by the formula:
10,000(C / W)(ri / rS)
in which C is the concentration, in mg per mL, of USP Pyrilamine Maleate RS in the Standard solution; W is the weight, in mg, of the Pyrilamine Maleate taken to prepare the Test solution; ri is the peak area response for each impurity; and rS is the response of the Standard solution: not more than 0.3% of any individual impurity is found, and not more than 1.0% of total impurities is found.
Assay—
Dissolve about 400 mg of Pyrilamine Maleate, previously dried and accurately weighed, in 50 mL of glacial acetic acid. Add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a blue-green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 20.07 mg of C17H23N3O·C4H4O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4493