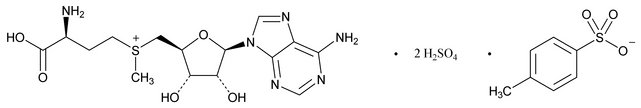
S-Adenosyl-l-methionine Disulfate Tosylate
C22H34N6O16S4 766.80
S-(Adenosyl)-l-methionine disulfate tosylate;
(3S)-5¢-[(3-Amino-3-carboxypropyl)methylsulfonio]-5¢-deoxyadenosine, disulfate-methylbenzenesulfonate

 [97540-22-2].
[97540-22-2].
Former Title: Ademetionine Disulfate Tosylate
C22H34N6O16S4 766.80
S-(Adenosyl)-l-methionine disulfate tosylate;
(3S)-5¢-[(3-Amino-3-carboxypropyl)methylsulfonio]-5¢-deoxyadenosine, disulfate-methylbenzenesulfonate
DEFINITION
S-Adenosyl-l-methionine Disulfate Tosylate is the disulfate–tosylate mixed salt of a mixture of diastereoisomers of the S-adenosyl-l-methionine ion. It contains NLT 95.0% and NMT 105.0% of S-adenosyl-l-methionine disulfate tosylate (C22H34N6O16S4) calculated through the content of S-adenosyl-l-methionine (C15H23N6O5S+), calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of S-adenosyl-l-methionine in the System suitability solution, as obtained in the Content of S-Adenosyl-l-Methionine.
COMPOSITION
• Content of S-Adenosyl-l-methionine
Solution A:
10 mL of glacial acetic acid in 500 mL of water. Add 2.06 g of sodium 1-hexanesulfonate, and dilute with water to 1000 mL.
Mobile phase:
Acetronitrile and Solution A (15:85)
System suitability solution:
400 µg/mL each of USP S-Adenosyl-l-methionine Disulfate Tosylate RS and USP S-Adenosyl-l-homocysteine RS
Standard solution A:
400 µg/mL of USP S-Adenosyl-l-homocysteine RS
Standard solution B:
200 µg/mL from Standard solution A
Standard solution C:
80 µg/mL from Standard solution A
Sample solution:
20 mg of S-Adenosyl-l-methionine Disulfate Tosylate in 40 mL of water. Stir for 30 min, then dilute with water to 50.0 mL. Transfer 1.0 mL of the solution to a 1.5-mL microcentrifuge tube, and centrifuge for 1 min. Use a portion of the supernatant.
Chromatographic system
Mode:
LC
Detector:
UV 260 nm
Column:
4.6-mm × 15-cm; 3-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution B
[Note—The relative retention times for S-adenosyl-l-homocysteine and S-adenosyl-l-methionine disulfate tosylate are about 0.68 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.5 between S-adenosyl-l-homocysteine and S-adenosyl-l-methionine
Tailing factor:
NMT 1.5, Standard solution B
Relative standard deviation:
NMT 2.0% for S-adenosyl-l-homocysteine, Standard solution B
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, and Sample solution
[Note—Record the chromatograms, and measure the area of the S-adenosyl-l-homocysteine peak in all three Standard solutions and the S-adenosyl-l-methionine disulfate tosylate peak in the Sample solution. ]
Plot a calibration curve of the peak area of the Standard solutions versus the corresponding S-adenosyl-l-homocysteine concentration, in mg/mL, and draw the straight line best fitting the three points. From the calibration curve, and using the peak area of S-adenosyl-l-methionine from the chromatogram from the Sample solution, determine the concentration, C, in mg/mL, of S-adenosyl-l-methionine as S-adenosyl-l-homocysteine in the Sample solution.
Calculate the percentage of C15H23N6O5S+ in the portion of S-Adenosyl-l-methionine Disulfate Tosylate taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak area of S-adenosyl-l-methionine from the Sample solution |
| rS | = | = peak area of S-adenosyl-l-homocysteine from the Standard solution |
| CS | = | = concentration of S-adenosyl-l-homocysteine in the Standard solution (mg/mL) |
| CU | = | = concentration of S-adenosyl-l-methionine in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of S-adenosyl-l-methionine, 399.44 |
| Mr2 | = | = molecular weight of S-adenosyl-l-homocysteine, 384.41 |
Acceptance criteria:
95.0%–105.0% on the anhydrous basis
• Content of Sulfate
Mobile phase:
8.0 mM sodium carbonate and 1.0 mM sodium bicarbonate in water
Standard solution:
0.18 mg/mL of potassium sulfate
Sample solution:
0.5 mg/mL of S-Adenosyl-l-methionine Disulfate Tosylate
Chromatographic system
Mode:
LC
Detector:
Ion detector with suppressed conductivity
Column:
4.0-mm × 25-cm; 7-µm packing L74
Column temperature:
30
Flow rate:
1 mL/min
Injection size:
25 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 8200 theoretical plates
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 1.0%
Analysis
Samples:
Standard solution and Sample solution
[Note—Measure the area of the sulfate peak. ]
Calculate the percentage of sulfate (SO4) in the portion of S-Adenosyl-l-methionine Disulfate Tosylate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of sulfate from the Sample solution |
| rS | = | = peak response of sulfate from the Standard solution |
| CS | = | = concentration of sulfate (SO4) in the Standard solution (mg/mL) |
| CU | = | = concentration of S-Adenosyl-l-methionine Disulfate Tosylate in the Sample solution (mg/mL) |
Acceptance criteria:
23.5%–26.5%
IMPURITIES
• Heavy Metals, Method I  231
231 :
NMT 20 ppm
:
NMT 20 ppm
SPECIFIC TESTS
• pH  791
791 :
1.0–2.0, in an aqueous solution (1 in 20)
:
1.0–2.0, in an aqueous solution (1 in 20)
• Water Determination, Method Ia  921
921 :
NMT 3.0%
:
NMT 3.0%
• Isomeric Ratio
Buffer:
Transfer 4.2 g of citric acid monohydrate and 2.03 g of sodium dihydrogen phosphate dihydrate to a 1-L volumetric flask, and dissolve in and dilute with water to volume.
Mobile phase:
4.0 g of sodium dodecyl sulfate and 440 mL of acetonitrile. Dilute with Buffer to 1 L.
Standard solution:
1.0 mg/mL of USP S-Adenosyl-l-methionine Disulfate Tosylate RS
Sample solution:
1.0 mg/mL of S-Adenosyl-l-methionine Disulfate Tosylate
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1.2 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for R,S-isomers and S,S-isomers are about 0.94 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.0 between the S,S-isomer and the R,S-isomer
Analysis
Samples:
Standard solution and Sample solution
Identify the peaks of the S,S- and R,S-isomers of the Sample solution by comparison with the Standard solution, and calculate the percentage of the S,S-isomer:
Result = [rSS/(rSS + rRS)] × 100
| rSS | = | = areas of the peaks corresponding to the S,S-isomer in the Sample solution |
| rRS | = | = areas of the peaks corresponding to the R,S-isomer in the Sample solution |
Acceptance criteria:
NLT 60% and NLT the labeled amount of the S,S-isomer
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store in a refrigerator.
• Labeling:
Label it to indicate the minimum content of S,S-isomer, as a percentage.
• USP Reference Standards  11
11
USP S-Adenosyl-l-methionine Disulfate Tosylate RS
USP S-Adenosyl-l-homocysteine RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1424
Pharmacopeial Forum: Volume No. 36(2) Page 438