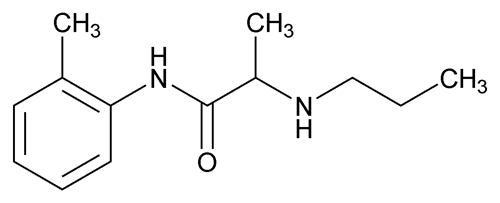
Prilocaine
(pril' oh kane).
Propranamide, N-(2-methylphenyl)-2-(propylamino)-.
2-(Propylamino)-o-propionotoluidide.
(RS)-N-(2-methylphenyl)-2-(propylamino)propanamide
» Prilocaine contains not less than 99.0 percent and not more than 101.0 percent of C13H20N2O, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers, and store below 25 .
.
USP Reference standards  11
11 —
—
USP Prilocaine Related Compound A RS
o-Toluidine hydrochloride.
CH3C6H4NH2HCl 143.62

 [CAS-636-21-5].
[CAS-636-21-5].
o-Toluidine hydrochloride.
CH3C6H4NH2HCl 143.62
USP Prilocaine Related Compound B RS
(RS)-N-(4-Methylphenyl)-2-(propylamino)propanamide.
C13H20N2O 220.31
(RS)-N-(4-Methylphenyl)-2-(propylamino)propanamide.
C13H20N2O 220.31
Identification,
Infrared Absorption  197K
197K —Because of the low melting point of prilocaine, the mortar, pestle, and potassium bromide must be at ambient temperature. Record the IR spectrum using the diffuse reflectance technique.
—Because of the low melting point of prilocaine, the mortar, pestle, and potassium bromide must be at ambient temperature. Record the IR spectrum using the diffuse reflectance technique.
Melting range, Class 1a  741
741 :
between 36
:
between 36 and 39
and 39 , without previous drying.
, without previous drying.
Water, Method Ia  921
921 :
not more than 0.5%, determined on 1.00 g of sample.
:
not more than 0.5%, determined on 1.00 g of sample.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Limit of prilocaine related compound A—
Mobile phase—
Prepare as directed under Related compounds.
Standard solution—
Dissolve an accurately weighed quantity of USP Prilocaine Related Compound A RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 1.3 µg per mL.
Test solution—
Transfer about 100 mg of Prilocaine, accurately weighed, to a 10-mL volumetric flask, dissolve and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
Use the system as described under Related compounds. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the signal-to-noise ratio of the major peak should be greater than 10.
)—
Use the system as described under Related compounds. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the signal-to-noise ratio of the major peak should be greater than 10.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks: any peak corresponding to prilocaine related compound A (o-toluidine) in the Test solution is not greater than the response of the major peak in the Standard solution (0.01%).
Related compounds—
Buffer—
Dilute 1.3 mL of a 1 M monobasic sodium phosphate solution (1.38 g diluted with water to 10 mL) and 32.5 mL of a 0.5 M anhydrous disodium hydrogen phosphate solution (7.1 g diluted with water to 100 mL) with water to 1 L. The pH of this solution is 8.0. Make adjustments as needed.
Mobile phase—
Prepare a degassed mixture of Buffer and acetonitrile (73:27). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve accurately weighed quantities of USP Prilocaine RS and USP Prilocaine Related Compound B RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having known concentrations of about 2.5 µg per mL and 3.0 µg per mL, respectively.
Test solution—
Transfer about 25 mg of Prilocaine, accurately weighed, to a 10-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 240-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.19 for prilocaine related compound B and 1.0 for prilocaine; the resolution, R, between prilocaine and prilocaine related compound B is not less than 3.0; and the signal-to-noise ratio for the prilocaine peak is not less than 10.
)—
The liquid chromatograph is equipped with a 240-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.19 for prilocaine related compound B and 1.0 for prilocaine; the resolution, R, between prilocaine and prilocaine related compound B is not less than 3.0; and the signal-to-noise ratio for the prilocaine peak is not less than 10.
Procedure—
Inject a volume (about 20 µL) of the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Run the chromatograms for at least 1.5 times the retention of prilocaine. Check the stability of the baseline by injecting Mobile phase. Calculate the percentage of each impurity in the portion of Prilocaine taken by the formula:
100(ri / rs)
in which ri is the individual peak response of each impurity; and rs is the sum of the responses of all the peaks: not more than 0.2% of any individual impurity is found; not more than one impurity exceeds 0.1%, and not more than 0.5% of total impurities is found.
Assay—
Dissolve 400 mg of Prilocaine, accurately weighed, in 50 mL of glacial acetic acid. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 perchloric acid is equivalent to 22.03 mg of C13H20N2O.
). Each mL of 0.1 perchloric acid is equivalent to 22.03 mg of C13H20N2O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4409
Pharmacopeial Forum: Volume No. 30(5) Page 1643