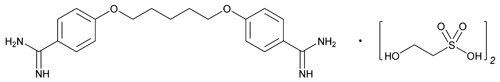
Pentamidine Isethionate
C19H24N4O2·(C2H6O4S)2 592.70
Ethanesulfonic acid, 2-hydroxy-, compd. with 4,4'-[1,5-pentanediylbis(oxy)]bis [benzenecarboximidamide];
4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide bis(2-hydroxyethanesulfonate)

 [140-64-7].
[140-64-7].
(pen tam' i deen eye'' se thye' oh nate).
C19H24N4O2·(C2H6O4S)2 592.70
Ethanesulfonic acid, 2-hydroxy-, compd. with 4,4'-[1,5-pentanediylbis(oxy)]bis [benzenecarboximidamide];
4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide bis(2-hydroxyethanesulfonate)
DEFINITION
Pentamidine Isethionate contains NLT 98.5% and NMT 101.5% of C19H24N4O2·(C2H6O4S)2, calculated on the dried basis.
IDENTIFICATION
• B. Oxygen-Flask Combustion  471
471
Barium chloride solution:
60 mg/mL of barium chloride in water
Analysis:
Burn 150 mg, using 10 mL of 3% hydrogen peroxide as the absorbing liquid. When the process is complete, acidify with 1 mL of diluted hydrochloric acid, and add 1 mL of the Barium chloride solution.
Acceptance criteria:
A white precipitate is formed.
• C.
The retention time of the pentamidine isethionate peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Organic Impurities.
ASSAY
• Procedure
Sample solution:
5 mg/mL in dimethylformamide. Add 0.25 mL of thymol blue TS.
Analysis:
Titrate under a stream of nitrogen with 0.1 M tetrabutylammonium hydroxide VS, determining the endpoint until the color changes to intense blue. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 29.63 mg of C19H24N4O2·(C2H6O4S)2.
). Each mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 29.63 mg of C19H24N4O2·(C2H6O4S)2.
Acceptance criteria:
98.5%–101.5% on the dried basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method I  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Residue on Ignition  281
281
Acceptance criteria:
NMT 0.1% on a 1-g sample
Organic Impurities
• Procedure
Buffer:
30 mg/mL of ammonium acetate in water, adjusted with triethylamine to a pH of 7.5
Mobile phase:
Methanol and Buffer (65:35)
System suitability solution:
Prepare 40.0 mL of a 2.5 mg/mL solution of USP Pentamidine Isethionate RS in water. Adjust with 0.2 M sodium hydroxide to a pH of 10.5, and boil under reflux for 20 min. Cool, and dilute with water to 50.0 mL. Transfer quantitatively 1 mL of this solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Standard solution:
2 µg/mL of USP Pentamidine Isethionate RS in Mobile phase
Sample solution:
1.0 mg/mL of Pentamidine Isethionate in Mobile phase. [Note—It must be demonstrated that the final product does not contain a detectable amount of alkyl 2-hydroxyethanesulphonates, a potential in-process impurity. ]
Chromatographic system
Mode:
LC
Detector:
UV 265 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
Run time:
3.5 times the retention time of pentamidine
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 2 between the two major peaks.
[Note—The chromatogram shows two major peaks. ]
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria
Individual impurities:
NMT 0.4%. [Note—Exclude any other peak producing a response of less than 0.02%. ]
Total impurities:
NMT 0.7%
SPECIFIC TESTS
• pH  791
791 :
4.5–6.5, in a carbon dioxide-free aqueous solution containing 50 mg/mL of Pentamidine Isethionate
:
4.5–6.5, in a carbon dioxide-free aqueous solution containing 50 mg/mL of Pentamidine Isethionate
• Loss on Drying  731
731 :
Dry at 105
:
Dry at 105 : it loses NMT 4.0% of its weight.
: it loses NMT 4.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light. Store at controlled room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4256
Pharmacopeial Forum: Volume No. 35(3) Page 570