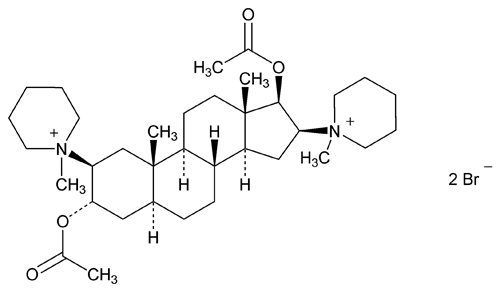
Pancuronium Bromide
(pan kure oh' nee um broe' mide).
Piperidinium, 1,1¢-[(2
1,1¢-(3
2
» Pancuronium Bromide contains not less than 98.0 percent and not more than 102.0 percent of C35H60Br2N2O4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers, protected from light and moisture. Store between 15 and 25
and 25 .
.
Identification—
B:
The RF value of the principal spot in the chromatogram of the Test solution corresponds to that of the chromatogram of Standard solution 2, as obtained in the test for Related compounds.
C:
A solution (1 in 10) meets the requirements of the tests for Bromide  191
191 .
.
Clarity of solution—
Hydrazine sulfate solution—
Transfer 1.0 g of hydrazine sulfate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Allow to stand for 4 to 6 hours before use.
Methenamine solution—
Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension—
[note—This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use. ] Transfer 25.0 mL of Hydrazine sulfate solution to the Methenamine solution in the 100-mL glass-stoppered flask. Mix, and allow to stand for 24 hours.
Opalescence standard—
[note—This suspension should not be used beyond 24 hours after preparation. ] Transfer 15.0 mL of the Primary opalescent suspension to a 1000-mL volumetric flask, dilute with water to volume, and mix.
Reference suspensions—
Transfer 5.0 mL of the Opalescence standard to a 100-mL volumetric flask, dilute with water to volume, and mix to obtain Reference suspension A. Transfer 10.0 mL of the Opalescence standard to a second 100-mL volumetric flask, dilute with water to volume, and mix to obtain Reference suspension B.
Test solution—
Dissolve 50 mg of Pancuronium Bromide in about 20 mL of water, dilute with water to 25 mL, and mix.
Procedure—
Transfer a sufficient portion of the Test solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15 to 25 mm to obtain a depth of 40 mm. Similarly transfer portions of Reference suspension A, Reference suspension B, and water to separate matching test tubes. Compare the Test solution, Reference suspension A, Reference suspension B, and water in diffused daylight, viewing vertically against a black background (see Visual Comparison under Spectrophotometry and Light-Scattering  851
851 ). [note—The diffusion of light must be such that Reference suspension A can readily be distinguished from water, and Reference suspension B can readily be distinguished from Reference suspension A. ] The Test solution shows the same or more clarity than Reference suspension A.
). [note—The diffusion of light must be such that Reference suspension A can readily be distinguished from water, and Reference suspension B can readily be distinguished from Reference suspension A. ] The Test solution shows the same or more clarity than Reference suspension A.
Color of solution—
Standard stock solution—
Prepare a solution of ferric chloride CS, cobaltous chloride CS, cupric sulfate CS, and a 10 g per L hydrochloric acid solution (3:3:2.4:1.6).
Standard solution—
[note—Prepare this solution just before use. ] Transfer 1 mL of Standard stock solution to a 100-mL volumetric flask, dilute with hydrochloric acid (10 g per L) solution to volume, and mix.
Test solution—
Use the Test solution prepared as directed in the test for Clarity of solution.
Procedure—
Transfer a sufficient portion of the Test solution to a test tube of colorless, transparent, neutral glass with a flat base and an external diameter of 15 to 25 mm to obtain a depth of 40 mm. Similarly transfer a portion of the Standard solution and water to separate matching test tubes. Compare the Test solution (see Visual Comparison under Spectrophotometry and Light-Scattering  851
851 ): the Test solution is not more intensely colored than the Standard solution or water.
): the Test solution is not more intensely colored than the Standard solution or water.
Water, Method I  921
921 :
not more than 8.0%.
:
not more than 8.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Related compounds—
Adsorbent:
0.2-mm layer of chromatographic silica gel mixture.
Test solution—
Prepare a solution of Pancuronium Bromide in methylene chloride containing 10 mg per mL.
Diluted test solution—
Dilute 1.0 mL of the Test solution with methylene chloride to 50 mL, and mix. Dilute 1.0 mL of the resulting solution with methylene chloride to 20 mL, and mix.
Standard solution 1—
Prepare a solution in methylene chloride containing 0.1 mg of USP Vecuronium Bromide RS and 10 mg of USP Pancuronium Bromide RS per mL.
Standard solution 2—
Prepare a solution of USP Pancuronium Bromide RS in methylene chloride containing 10 mg per mL.
Developing solvent system:
a mixture of isopropyl alcohol, acetonitrile, and a 400 g per L solution of sodium iodide (85:10:5).
Procedure—
Apply separately 5-µL portions of the Test solution, Diluted test solution, Standard solution 1, and Standard solution 2 to a suitable thin-layer chromatographic plate (see Thin-Layer Chromatography under Chromatography  621
621 ). Develop in an unlined and unsaturated tank over a path of 8 cm. Spray the plate with a 20 g per L solution of sodium nitrite, and allow to dry for 5 minutes. Spray the plate with Dragendorff's TS, and cover the plate with a transparent glass cover. Any spot in the chromatogram obtained from the Test solution due to vecuronium bromide is not more intense than the corresponding spot in the chromatogram obtained from Standard solution 1: equivalent to not more than 1.0% of vecuronium bromide. Any other spot in the chromatogram obtained from the Test solution, except for the principal spot and any spot due to vecuronium bromide, is not more intense than the spot in the chromatogram obtained from the Diluted test solution: equivalent to not more than 0.1% of any individual impurity. In a valid test, the chromatogram obtained from Standard solution 1 shows two clearly separated spots. The RF values for pancuronium bromide and vecuronium bromide are about 0.5 and 0.64, respectively.
). Develop in an unlined and unsaturated tank over a path of 8 cm. Spray the plate with a 20 g per L solution of sodium nitrite, and allow to dry for 5 minutes. Spray the plate with Dragendorff's TS, and cover the plate with a transparent glass cover. Any spot in the chromatogram obtained from the Test solution due to vecuronium bromide is not more intense than the corresponding spot in the chromatogram obtained from Standard solution 1: equivalent to not more than 1.0% of vecuronium bromide. Any other spot in the chromatogram obtained from the Test solution, except for the principal spot and any spot due to vecuronium bromide, is not more intense than the spot in the chromatogram obtained from the Diluted test solution: equivalent to not more than 0.1% of any individual impurity. In a valid test, the chromatogram obtained from Standard solution 1 shows two clearly separated spots. The RF values for pancuronium bromide and vecuronium bromide are about 0.5 and 0.64, respectively.
Assay—
Transfer about 200 mg of Pancuronium Bromide, accurately weighed, to a 100-mL beaker, and dissolve by stirring in 50 mL of acetic anhydride. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 36.63 mg of C35H60Br2N2O4.
). Each mL of 0.1 N perchloric acid is equivalent to 36.63 mg of C35H60Br2N2O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4207
Pharmacopeial Forum: Volume No. 32(1) Page 130