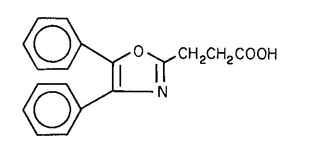
Oxaprozin
(ox'' a proe' zin).
2-Oxazolepropanoic acid, 4,5-diphenyl-.
4,5-Diphenyl-2-oxazolepropionic acid
» Oxaprozin contains not less than 98.5 percent and not more than 101.5 percent of C18H15NO3, calculated on the dried basis.
[note—Because of light sensitivity, protect all oxaprozin samples and Standard solutions from light. ]
Packaging and storage—
Preserve in tight, light-resistant containers, and store at controlled room temperature.
Identification—
A:
Infrared Absorption  197K
197K : previously dried at 105
: previously dried at 105 for 2 hours.
for 2 hours.
B:
Ultraviolet Absorption  197U
197U : previously dried at 105
: previously dried at 105 for 2 hours. The absorbance of the sample at 285 nm is between 0.455 and 0.495.
for 2 hours. The absorbance of the sample at 285 nm is between 0.455 and 0.495.
Solution:
10 µg per mL.
Medium:
methanol.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 0.3% of its weight.
for 2 hours: it loses not more than 0.3% of its weight.
Residue on ignition  281
281 :
not more than 0.3%.
:
not more than 0.3%.
Arsenic, Method II  211
211 :
1 µg per g.
:
1 µg per g.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Chromatographic purity—
Solution A:
0.1% phosphoric acid adjusted with phosphoric acid to a pH of 2.00 ± 0.1.
Solution B—
Use acetonitrile.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Diluent A:
a mixture of acetonitrile, methylene chloride, and water (48:1:1).
Diluent B:
a mixture of acetonitrile and water (1:1).
Standard stock solution—
Dissolve an accurately weighed quantity of USP Oxaprozin RS in acetonitrile to obtain a solution having a concentration of about 200 µg per mL.
Standard solution—
Transfer 5.0 mL of Standard stock solution to a 200-mL volumetric flask, and dilute with Diluent A to volume.
Benzil solution:
200 µg of benzil per mL in acetonitrile.
Resolution solution—
Transfer 5.0 mL of Benzil solution and 5.0 mL of Standard stock solution to a 100-mL volumetric flask, and dilute with Diluent A to volume to obtain a solution having known concentrations of about 10 µg of each per mL.
Test solution A—
[note—Test solution A is used to monitor all known and unknown impurities, except imidazolic acid and oximide. ] Transfer about 100 mg of Oxaprozin, accurately weighed, to a 100-mL volumetric flask; add 2 mL of methylene chloride, 2 mL of water, and 75 mL of acetonitrile; and sonicate after each solvent is added. Dilute with acetonitrile to volume.
Test solution B—
[note—Test solution B is used to monitor only imidazolic acid and oximide. ] Transfer about 100 mg of Oxaprozin, accurately weighed, to a 100-mL volumetric flask; add 75 mL of Diluent B to dissolve the Oxaprozin; and dilute with Diluent B to volume.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 238-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L7. The flow rate is 1.0 mL per minute. The chromatograph is programmed as follows.
)—
The liquid chromatograph is equipped with a 238-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L7. The flow rate is 1.0 mL per minute. The chromatograph is programmed as follows.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
|---|---|---|---|
| 0 | 70 | 30 | equilibration |
| 0–20 | 70 | 30 | isocratic |
| 21–60 | 70®0 | 30®100 | linear gradient |
| 60–61 | 0®70 | 100®30 | linear gradient |
| 61–70 | 70 | 30 | isocratic |
Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.1 for benzil and 1.0 for oxaprozin; and the resolution, R, between oxaprozin and benzil is not less than 3.0. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0, and the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Inject 20 µL of Test solution A and Test solution B into the chromatograph, record the chromatogram, and measure the areas for all the peaks. Calculate the percentage of each impurity in the portion of Oxaprozin taken by the formula:
100(Fri / rs)
in which F is the relative response factor and is equal to 1.15 for the imidazolic acid peak with a relative retention time of 0.14, 1.21 for any peak with a relative retention time of 0.42, 0.91 for the oximide peak with a relative retention time of 0.73, 0.85 for any peak with a relative retention time of 0.84, 1.29 for any peak with a relative retention time of 1.08, 1.46 for any peak with a relative retention time of 1.50, and 2.09 for any peak with a relative retention time of 1.57; ri is the peak response for each impurity; and rs is the sum of the responses of all the peaks: not more than 0.1% of any individual impurity is found, and not more than 0.5% of total impurities is found. [note—The values of F for all known impurities except imidazolic acid and oximide were found using Test solution A, and the values of F for imidazolic acid and oximide were found using Test solution B. ]
Assay—
Dissolve about 400 mg of Oxaprozin, previously dried at 105 for 2 hours and accurately weighed, in about 100 mL of alcohol in a narrow-mouth container, and titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically (see Titrimetry
for 2 hours and accurately weighed, in about 100 mL of alcohol in a narrow-mouth container, and titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically (see Titrimetry  541
541 ). Each mL of 0.1 N sodium hydroxide is equivalent to 29.332 mg of oxaprozin.
). Each mL of 0.1 N sodium hydroxide is equivalent to 29.332 mg of oxaprozin.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4154
Pharmacopeial Forum: Volume No. 32(1) Page 130