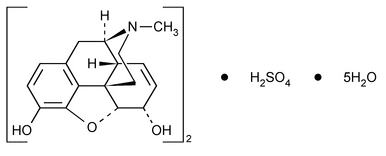Morphine Sulfate
(mor' feen sul' fate).
(C17H19NO3)2·H2SO4·5H2O




 758.83
758.83
Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl, (5 ,6
,6 )-, sulfate (2:1) (salt), pentahydrate.
)-, sulfate (2:1) (salt), pentahydrate.
7,8-Didehydro-4,5 -epoxy-17-methylmorphinan-3,6
-epoxy-17-methylmorphinan-3,6 -diol sulfate (2:1) (salt) pentahydrate
-diol sulfate (2:1) (salt) pentahydrate


 [6211-15-0].
[6211-15-0].
Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl, (5
7,8-Didehydro-4,5
Anhydrous 668.77
» Morphine Sulfate contains not less than 98.0 percent and not more than 102.0 percent of (C17H19NO3)2·H2SO4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight, light-resistant containers. Store up to 40 as permitted by the manufacturer.
as permitted by the manufacturer.
Identification—
A:
Infrared Absorption  197K
197K : dried at 145
: dried at 145 for 1 hour.
for 1 hour.
B:
To 1 mg in a porcelain crucible or small dish add 0.5 mL of sulfuric acid containing, in each mL, 1 drop of formaldehyde TS: an intense purple color is produced at once, and quickly changes to deep blue-violet (distinction from codeine, which gives at once an intense violet-blue color, and from hydromorphone, which gives at first a yellow to brown color, changing to pink and then to purplish red).
C:
To a solution of 5 mg in 5 mL of sulfuric acid in a test tube add 1 drop of ferric chloride TS, mix, and heat in boiling water for 2 minutes: a blue color is produced, and when 1 drop of nitric acid is added, it changes to dark red-brown (codeine and ethylmorphine give the same color reactions, but hydromorphone and papaverine do not produce this color change).
D:
A solution (1 in 50) responds to the tests for Sulfate  191
191 .
.
Specific rotation  781S
781S :
between
:
between  107
107 and
and  109.5
109.5 .
.
Test solution:
the equivalent of 20 mg per mL, in water.
Acidity—
Dissolve 500 mg in 15 mL of water, add 1 drop of methyl red TS, and titrate with 0.020 N sodium hydroxide: not more than 0.50 mL is required to produce a yellow color.
Water, Method I  921
921 :
between 10.4% and 13.4% is found.
:
between 10.4% and 13.4% is found.
Residue on ignition  281
281 :
not more than 0.1%, from 500 mg.
:
not more than 0.1%, from 500 mg.
Chloride—
To 10 mL of a solution (1 in 100) add 1 mL of 2 N nitric acid and 1 mL of silver nitrate TS: no precipitate or turbidity is produced immediately.
Ammonium salts—
Heat 200 mg with 5 mL of 1 N sodium hydroxide on a steam bath for 1 minute: no odor of ammonia is perceptible.
Limit of foreign alkaloids—
Dissolve 1.00 g in 10 mL of 1 N sodium hydroxide in a separator, and shake the solution with three successive portions of 15, 10, and 10 mL of chloroform, passing the chloroform solutions through a small filter previously moistened with chloroform. Shake the combined chloroform solutions with 5 mL of water, separate the chloroform layer, and carefully evaporate on a steam bath to dryness. To the residue add 10.0 mL of 0.020 N sulfuric acid, and heat gently until dissolved. Cool, add 2 drops of methyl red TS, and titrate the excess acid with 0.020 N sodium hydroxide: not less than 7.5 mL is required (1.5%).
Assay—
Mobile phase—
Dissolve 0.73 g of sodium 1-heptanesulfonate in 720 mL of water, add 280 mL of methanol and 10 mL of glacial acetic acid, mix, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Morphine Sulfate RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 0.24 mg per mL. Prepare a fresh solution daily.
System suitability preparation—
Dissolve suitable quantities of USP Morphine Sulfate RS and phenol in Mobile phase to obtain a solution containing about 0.24 and 0.15 mg per mL, respectively.
Assay preparation—
Transfer about 24 mg of Morphine Sulfate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 284-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation and the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.7 for phenol and 1.0 for morphine sulfate; the resolution, R, between phenol and morphine sulfate is not less than 2.0; the tailing factor for the morphine sulfate peak is not more than 2.0; and the relative standard deviation for replicate injections of the Standard preparation is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 284-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation and the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.7 for phenol and 1.0 for morphine sulfate; the resolution, R, between phenol and morphine sulfate is not less than 2.0; the tailing factor for the morphine sulfate peak is not more than 2.0; and the relative standard deviation for replicate injections of the Standard preparation is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 25 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of (C17H19NO3)2·H2SO4 in the portion of Morphine Sulfate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of anhydrous morphine sulfate in the Standard preparation, as determined from the concentration of USP Morphine Sulfate RS corrected for moisture content by a titrimetric water determination; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3955
Pharmacopeial Forum: Volume No. 30(5) Page 1639