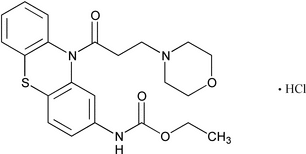
Moricizine Hydrochloride
(mor i' si zeen hye'' droe klor' ide).
Carbamic acid, [10-[3-(4-morpholinyl)-l-oxopropyl]-10H-phenothiazin-2-yl]-, ethyl ester, hydrochloride.
Ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate, hydrochloride
» Moricizine Hydrochloride contains not less than 98.0 percent and not more than 102.0 percent of C22H25N3O4S·HCl, calculated on the anhydrous and alcohol-free basis.
Packaging and storage—
Preserve in tight containers.
Identification—
C:
Prepare a test solution in methanol containing 20 mg of Moricizine Hydrochloride per mL. Similarly prepare a Standard solution in methanol containing 20 mg ofUSP Moricizine Hydrochloride RS. Separately apply 5 µL of each solution on a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a chromatographic chamber lined with filter paper saturated with a solvent system consisting of a mixture of chloroform, methanol, and diethylamine (91:7:2). Develop the chromatogram, protected from light, until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber. Spray the plate with a freshly prepared ferric chloride solution prepared by adding 20 mL of 10% ferric chloride solution to 200 mg of potassium ferricyanide dissolved in 20 mL of water: the RF value of the blue spot in the chromatogram obtained from the test solution corresponds to that in the chromatogram obtained from the Standard solution.
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a chromatographic chamber lined with filter paper saturated with a solvent system consisting of a mixture of chloroform, methanol, and diethylamine (91:7:2). Develop the chromatogram, protected from light, until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber. Spray the plate with a freshly prepared ferric chloride solution prepared by adding 20 mL of 10% ferric chloride solution to 200 mg of potassium ferricyanide dissolved in 20 mL of water: the RF value of the blue spot in the chromatogram obtained from the test solution corresponds to that in the chromatogram obtained from the Standard solution.
Clarity of solution—
Dissolve 1 g in 30 mL of methanol, sonicating for 5 minutes if necessary. The solution is not less clear than an equal volume of methanol contained in a similar vessel and examined similarly.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 4 hours: it loses not more than 1.0% of its weight.
for 4 hours: it loses not more than 1.0% of its weight.
Water, Method I  921
921 :
not more than 1.0%.
:
not more than 1.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
10 µg per g.
:
10 µg per g.
Chromatographic purity—
Mobile phase—
Prepare a mixture of water, acetonitrile, and triethylamine (580:420:1) containing 0.005 M sodium 1-octane sulfonate, and adjust with glacial acetic acid to a pH of 4.2. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Diluent—
Prepare a mixture of 0.02 N hydrochloric acid and acetonitrile (58:42).
Internal standard solution—
Prepare a solution of butamben in Diluent containing about 0.1 mg per mL.
Standard solution—
Prepare a solution ofUSP Moricizine Hydrochloride RS in Diluent having a known concentration of about 0.10 mg per mL. Transfer 10.0 mL of this solution to a 500-mL volumetric flask, add 25.0 mL of Internal standard solution, dilute with Diluent to volume, and mix to obtain a solution containing about 0.0020 mg ofUSP Moricizine Hydrochloride RS per mL. [note—Protect this solution from light. ]
Test solution—
Transfer about 100 mg of Moricizine Hydrochloride, accurately weighed, to a 100-mL low-actinic volumetric flask, add 5.0 mL of Internal standard solution, dilute with Diluent to volume, and mix. [note—Protect this solution from light. ]
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and is maintained at a constant temperature of about 35
)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and is maintained at a constant temperature of about 35 . The flow rate is about 2.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.6 for moricizine and 1.0 for butamben; the resolution, R, between the moricizine peak and the butamben peak is not less than 2; and the relative standard deviation for replicate injections is not more than 5%.
. The flow rate is about 2.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.6 for moricizine and 1.0 for butamben; the resolution, R, between the moricizine peak and the butamben peak is not less than 2; and the relative standard deviation for replicate injections is not more than 5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms for a period of time that is five times the elution time of moricizine, and measure the responses for the peaks, except for the solvent peak. Calculate the percentage of each impurity peak in the portion of Moricizine Hydrochloride taken by the formula:
100C(Ri / RS)
in which C is the concentration, in mg per mL, ofUSP Moricizine Hydrochloride RS in the Standard solution, Ri is the ratio of the peak areas of an individual impurity peak to the butamben peak obtained from the Test solution, and RS is the ratio of the peak areas of the moricizine peak to the butamben peak obtained from the Standard solution. Any impurity eluting before the moricizine peak is not more than 0.25%, any impurity eluting after the moricizine peak is not more than 0.20%, and the total of all impurities is not more than 1.5%, any impurity of less than 0.1% being disregarded.
Limit of alcohol (C2H5OH)—
Standard solution—
Transfer 6.0 mL of dehydrated alcohol to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a second 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a third 100-mL volumetric flask, dilute with water to volume, and mix. This solution contains 0.1184 mg of C2H5OH per mL.
Test solution—
Transfer about 1 g of Moricizine Hydrochloride, accurately weighed, to a 50-mL glass-stoppered centrifuge tube, add 19.0 mL of water, and sonicate to dissolve. Transfer 1.0 mL of 3 N ammonium hydroxide to the tube, insert the stopper, and shake the tube by mechanical means for 30 minutes. Centrifuge, draw off a portion of the clear supernatant and filter through a filter having a porosity of 0.5 µm or finer.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.8-m glass column that contains support S2. The column is maintained at 150
)—
The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.8-m glass column that contains support S2. The column is maintained at 150 , and the injection port and detector block are maintained at 170
, and the injection port and detector block are maintained at 170 . Helium is used as the carrier gas at a flow rate of about 50 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 3%.
. Helium is used as the carrier gas at a flow rate of about 50 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 3%.
Procedure—
Separately inject equal volumes (about 5 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of C2H5OH in the portion of Moricizine Hydrochloride taken by the formula:
2(C/W)(rU / rS)
in which C is the concentration, in mg per mL, of C2H5OH in the Standard solution; W is the weight, in g, of Moricizine Hydrochloride taken to prepare the Test solution; and rU and rS are the alcohol peak responses obtained from the Test solution and the Standard solution, respectively. Not more than 0.25% is found.
Content of chloride—
Transfer about 400 mg of Moricizine Hydrochloride, accurately weighed, to a conical flask, add 75 mL of methanol, and swirl to dissolve. Add 5 mL of glacial acetic acid, and three drops of eosin Y TS, and titrate with 0.1 N silver nitrate VS to a pink endpoint. Each mL of 0.1 N silver nitrate is equivalent to 3.546 mg of Cl. Not less than 7.49% and not more than 7.80% is found, calculated on the anhydrous and alcohol-free basis.
Assay—
Mobile phase—
Prepare a mixture of water, acetonitrile, glacial acetic acid, and triethylamine (580:420:20:1) containing 0.005 M sodium 1-octane sulfonate. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Diluent—
Prepare a mixture of 0.02 N hydrochloric acid and acetonitrile (58:42).
Internal standard solution—
Prepare a solution of butamben in Diluent containing about 5 mg per mL.
Standard preparation—
Transfer about 25 mg ofUSP Moricizine Hydrochloride RS, accurately weighed, to a 25-mL low-actinic volumetric flask, add 5.0 mL of Internal standard solution, dilute with Diluent to volume, and mix. [note—Protect this solution from light. ]
Assay preparation—
Transfer about 1 mg per mL of Moricizine Hydrochloride, accurately weighed, to a 50-mL low-actinic volumetric flask, add 10.0 mL of Internal standard solution, dilute with Diluent to volume, and mix. [note—Protect this solution from light. ]
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and is maintained at a constant temperature of about 35
)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and is maintained at a constant temperature of about 35 . The flow rate is about 2.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.6 for moricizine, 1.7 for the reverse Mannich product, 2.0 for the amide hydrolysis product, and 1.0 for butamben; the resolution, R, between the moricizine peak and the butamben peak is not less than 2; and the relative standard deviation for replicate injections is not more than 2%.
. The flow rate is about 2.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.6 for moricizine, 1.7 for the reverse Mannich product, 2.0 for the amide hydrolysis product, and 1.0 for butamben; the resolution, R, between the moricizine peak and the butamben peak is not less than 2; and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C22H25N3O4S·HCl in the portion of Moricizine Hydrochloride taken by the formula:
50C(RU / RS)
in which C is the concentration, in mg per mL, ofUSP Moricizine Hydrochloride RS in the Standard preparation; and RU and RS are the ratios of the peak area responses of moricizine and butamben obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3952
Pharmacopeial Forum: Volume No. 28(3) Page 774